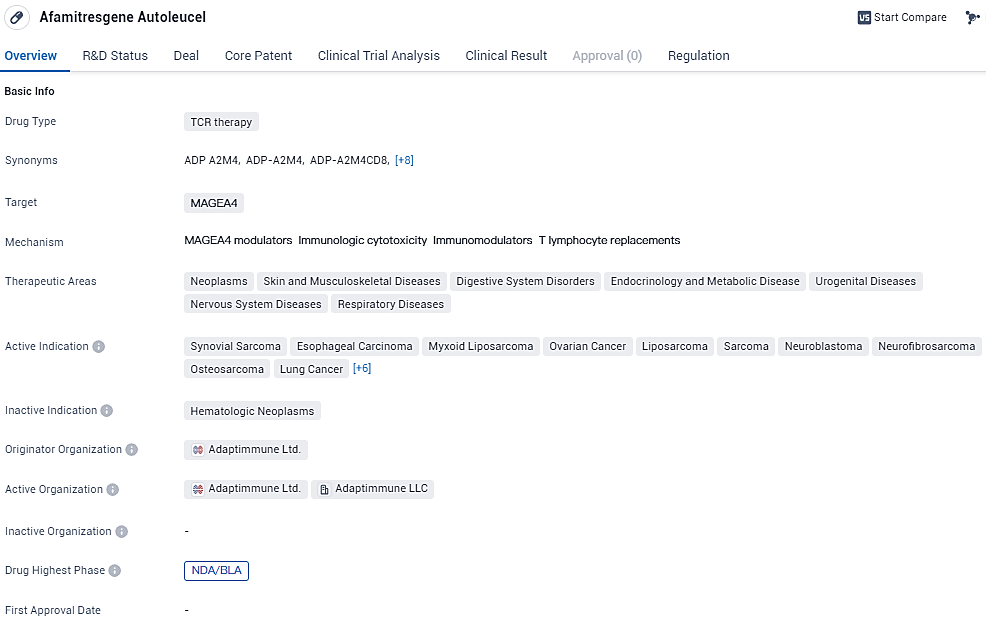
FDA Approves Adaptimmune's Afami-cel Submission for Advanced Synovial Sarcoma with Expedited Review
Adaptimmune Therapeutics plc, an enterprise revolutionizing solid tumor cancer care via cellular therapeutic approaches, has disclosed that its application to license afami-cel – a pioneering form of gene-modified T-cell treatment for progressive synovial sarcoma – has gained the approval of the U.S. Food and Drug Administration for expedited examination. The submission is subject to the Prescription Drug User Fee Act with an anticipated decision deadline set for August 4, 2024.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Adrian Rawcliffe, CEO of Adaptimmune, remarked, “Receiving the green light from the FDA for our BLA is a milestone that edges us closer to transforming the paradigm for treating synovial sarcoma patients. We believe our product line is poised for success and, upon authorization, possess both the financial resources and operational infrastructure to introduce afami-cel to the market as the pioneering engineered T-cell therapy for solid tumors.”
Dennis Williams, PharmD, and Senior Vice President of Late-Stage Development, observed, “The prognosis for advanced synovial sarcoma traditionally has been grim, with subsequent treatment options achieving minimal response rates and a median survival under one year post two rounds of therapy.”
The FDA's nod is bolstered by encouraging outcomes from the first cohort of the pivotal SPEARHEAD-1 study, which achieved its main goal of demonstrating effectiveness. These significant findings were unveiled at the 2023 Annual Meeting of the Connective Tissue Oncology Society.
Afami-cel, differentiated by engineered T-cell receptors targeting MAGEA4, is crafted as a one-time therapeutic intervention for those battling advanced synovial sarcoma. The most recent therapeutic agent in this context granted approval by the FDA before this was Votrient, almost a decade ago in 2012.
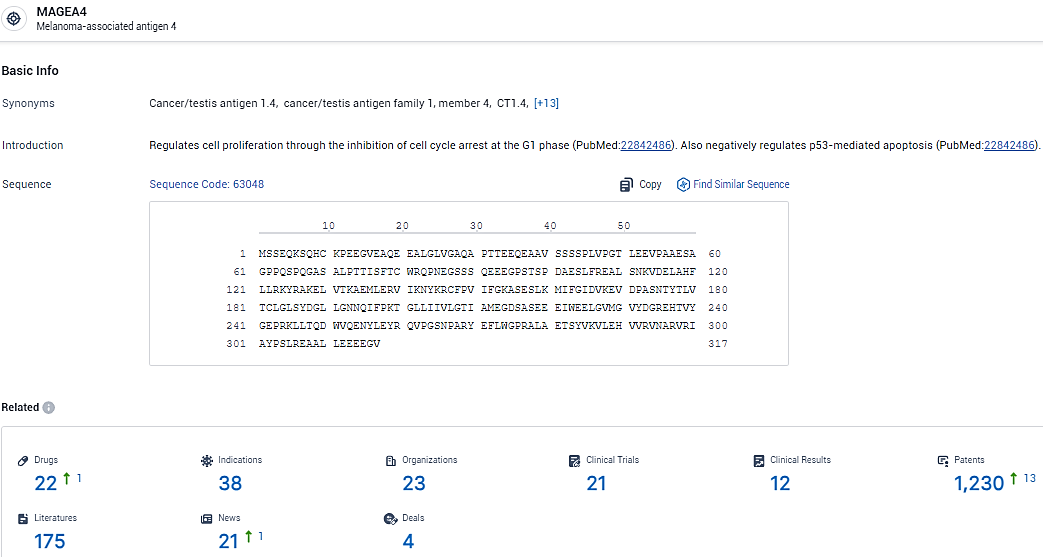
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 7, 2024, there are 22 investigational drugs for the MAGEA4 target, including 38 indications, 23 R&D institutions involved, with related clinical trials reaching 21, and as many as 1230 patents.
Afami-cel targets MAGEA4 and has shown potential in treating various types of cancer and related diseases. With its active indications covering a wide range of cancers, the drug has reached the highest phase of development and is currently undergoing regulatory review with priority status. The designations it has received further emphasize its potential as an innovative and much-needed treatment option in the field of biomedicine.