Tectonic Therapeutic Secures US IND Approval for TX45 Lead Program
Tectonic Therapeutic, Inc., a biotech firm in the clinical trial phase that specializes in discovering and developing therapeutic proteins and antibodies targeting G-protein coupled receptors, has announced that the U.S. Food and Drug Administration has approved its Investigational New Drug application for TX45. This Fc-relaxin fusion protein is being studied for its potential to treat patients with Group 2 PH-HFpEF.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
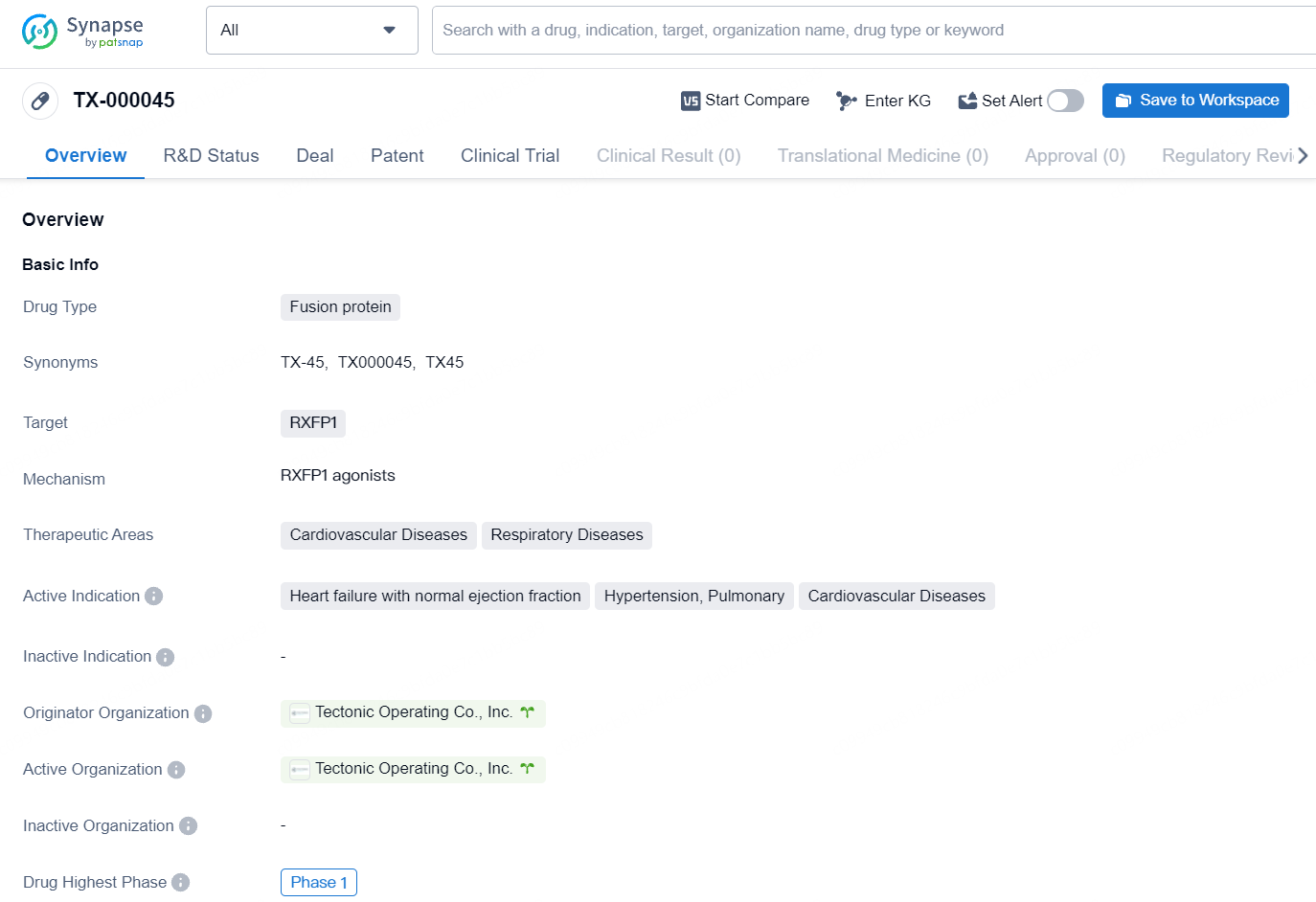
TX45 is focused on correcting the physiological irregularities of PH-HFpEF by promoting pulmonary and systemic vasodilation, improving cardiac diastolic function, and potentially remodeling both pulmonary vessels and cardiac muscle. This could result in a significant enhancement of exercise capacity in affected patients.
"Our innovative Fc-relaxin fusion protein, TX45, has been designed to enhance its pharmacological profile. The safety, pharmacokinetic, and pharmacodynamic information we have collected so far supports the launch of our Phase 2 clinical trial for our target patient group," said Alise Reicin, M.D., President and CEO of Tectonic.
"We anticipate releasing top-line results from our Phase 1a single-dose healthy volunteer trial in September and from our Phase 1b single-dose patient trial by mid-2025. We are optimistic about TX45’s potential to meet the unmet needs of patients with PH-HFpEF, who currently have no approved treatment options," Reicin added.
Tectonic plans to start the global Phase 2 clinical trial of TX45 for PH-HFpEF in the third quarter of 2024, with preliminary results expected in 2026. This study aims to assess the efficacy in a broad population of PH-HFpEF patients and will also focus on Group 2 PH patients with a more severe form of the disease, characterized by a baseline pulmonary vascular resistance greater than 3 Wood units.
The trial intends to enroll up to 180 participants, who will be randomly assigned to one of two TX45 dosage regimens or a placebo. TX45 will be administered via subcutaneous injection over a period of 24 weeks, followed by an 8-week follow-up. Primary and secondary endpoints will include changes from baseline in PVR and other relevant hemodynamic measures. The trial will also examine changes in six-minute walk distance due to TX45.
Tectonic’s leading program, TX000045 (TX45), comprises an Fc-relaxin fusion protein with optimized pharmacokinetics and biophysical characteristics that activates the RXFP1 receptor, which is the GPCR target of the hormone relaxin. Relaxin, normally present at low levels in both men and women, is upregulated during pregnancy. It facilitates vasodilation, decreases systemic and pulmonary vascular resistance, and boosts cardiac output to meet the increased need for oxygen and nutrients by the developing fetus. Additionally, relaxin has anti-fibrotic effects on pelvic ligaments to assist in childbirth.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
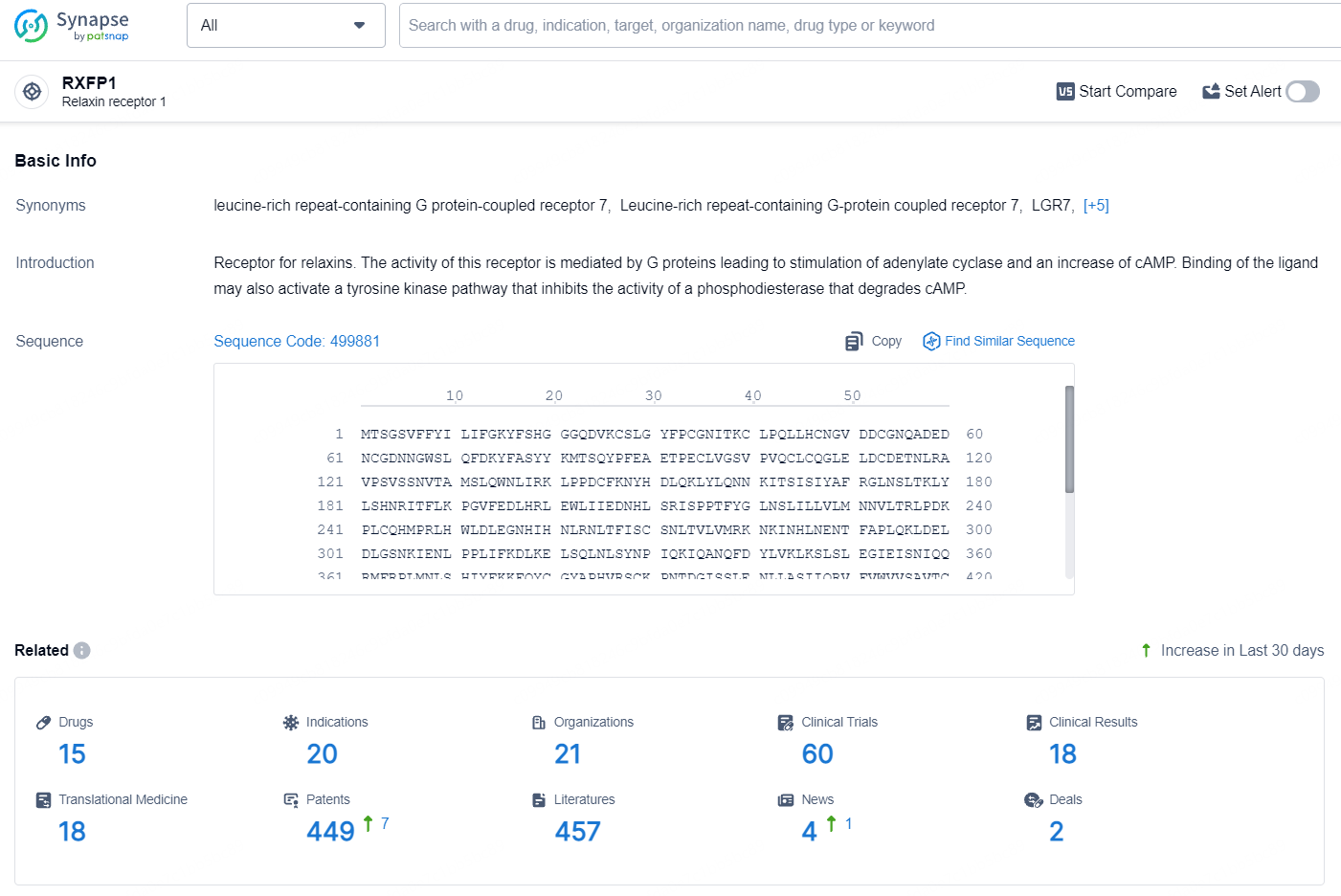
According to the data provided by the Synapse Database, As of August 5, 2024, there are 15 investigational drugs for the RXFP1 target, including 20 indications, 21 R&D institutions involved, with related clinical trials reaching 60, and as many as 449 patents.
TX-000045 is a fusion protein drug in Phase 1 development, targeting RXFP1 to address cardiovascular and respiratory diseases. Its originator organization is Tectonic Operating Co., Inc. Further research and development will be necessary to fully evaluate its potential as a therapeutic option in the specified therapeutic areas.