Review and Recent Advancements in Aldosterone Antagonists
The main function of Aldosterone is to promote the reabsorption of sodium ions and excretion of potassium in the epithelial cells of the renal distal convoluted tubule and collecting duct, i.e., sodium preservation and potassium excretion. While reabsorbing sodium, it also osmotically reabsorbs water, thereby maintaining extracellular fluid volume and blood volume. Aldosterone can also act on sweat gland, salivary gland ducts, and gastrointestinal epithelial cells, reducing sodium ion excretion and increasing potassium excretion in sweat, saliva, and feces through sodium preservation and potassium excretion.
Aldosterone can enhance the sensitivity of vascular smooth muscle to vasoconstrictive substances, indirectly causing other diseases in the body. Excessive aldosterone secretion can lead to sodium and water retention, causing high blood sodium, low blood potassium, alkalosis, and even persistent hypertension; conversely, low aldosterone secretion can lead to excessive excretion of sodium and water, resulting in high blood sodium, low blood potassium, acidosis or hypotension.
The renin-angiotensin-aldosterone system plays a key role in the onset and development of many diseases. Therefore, blocking or inhibiting any stage in this mechanism can achieve the purpose of treating diseases, making aldosterone antagonists a subject of great interest.
Aldosterone Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 3 Sep 2023, there are a total of 4 Aldosterone drugs worldwide, from 5 organizations, covering 8 indications, and conducting 10 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.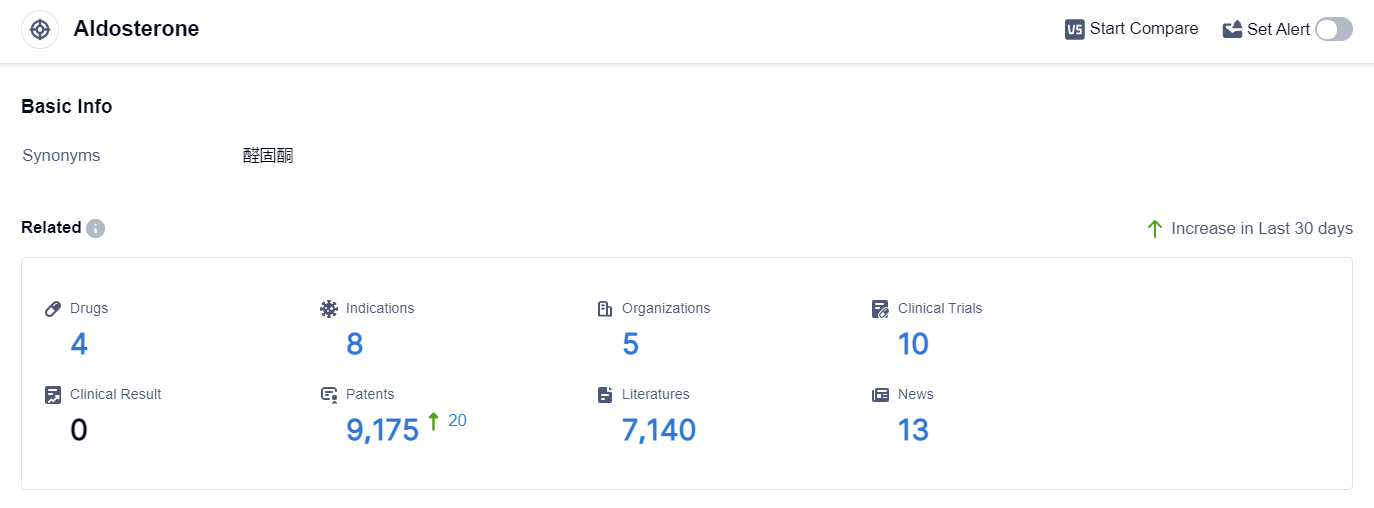
The analysis of the target Aldosterone reveals a competitive landscape with multiple companies involved in its development. Pfizer Inc. is leading with an approved drug, while Mayo Clinic is actively conducting research in Phase 1/2 and Phase 1. Japan, the United States, and South Africa are the countries/locations showing progress in the development of Aldosterone-related treatments.
The indications for Aldosterone-related drugs include Hyperaldosteronism, Edema, Cardiac, Acute congestive heart failure, and Hypertension. The presence of small molecule drugs, biological products, and an unknown drug type indicates diverse approaches to targeting Aldosterone.
Overall, the analysis suggests a promising future for the development of Aldosterone-targeted therapies, with potential advancements in addressing various medical needs associated with Aldosterone-related conditions.
Key Drug: Potassium Canrenoate
Potassium Canrenoate is a small molecule drug that targets aldosterone and is used in the treatment of cardiovascular diseases, endocrinology and metabolic diseases, and other related conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug is indicated for the treatment of acute congestive heart failure, edema (cardiac), hyperaldosteronism, and edema. Acute congestive heart failure refers to a sudden onset of heart failure symptoms, such as shortness of breath and fluid retention. Edema, both cardiac and non-cardiac, refers to the accumulation of fluid in the body's tissues, leading to swelling. Hyperaldosteronism is a condition characterized by excessive production of aldosterone, which can lead to high blood pressure and fluid retention.
Potassium Canrenoate received its first global approval in September 1967. It was developed by G.D. Searle & Co., Inc., and its approval signifies its efficacy and safety in treating the mentioned indications.