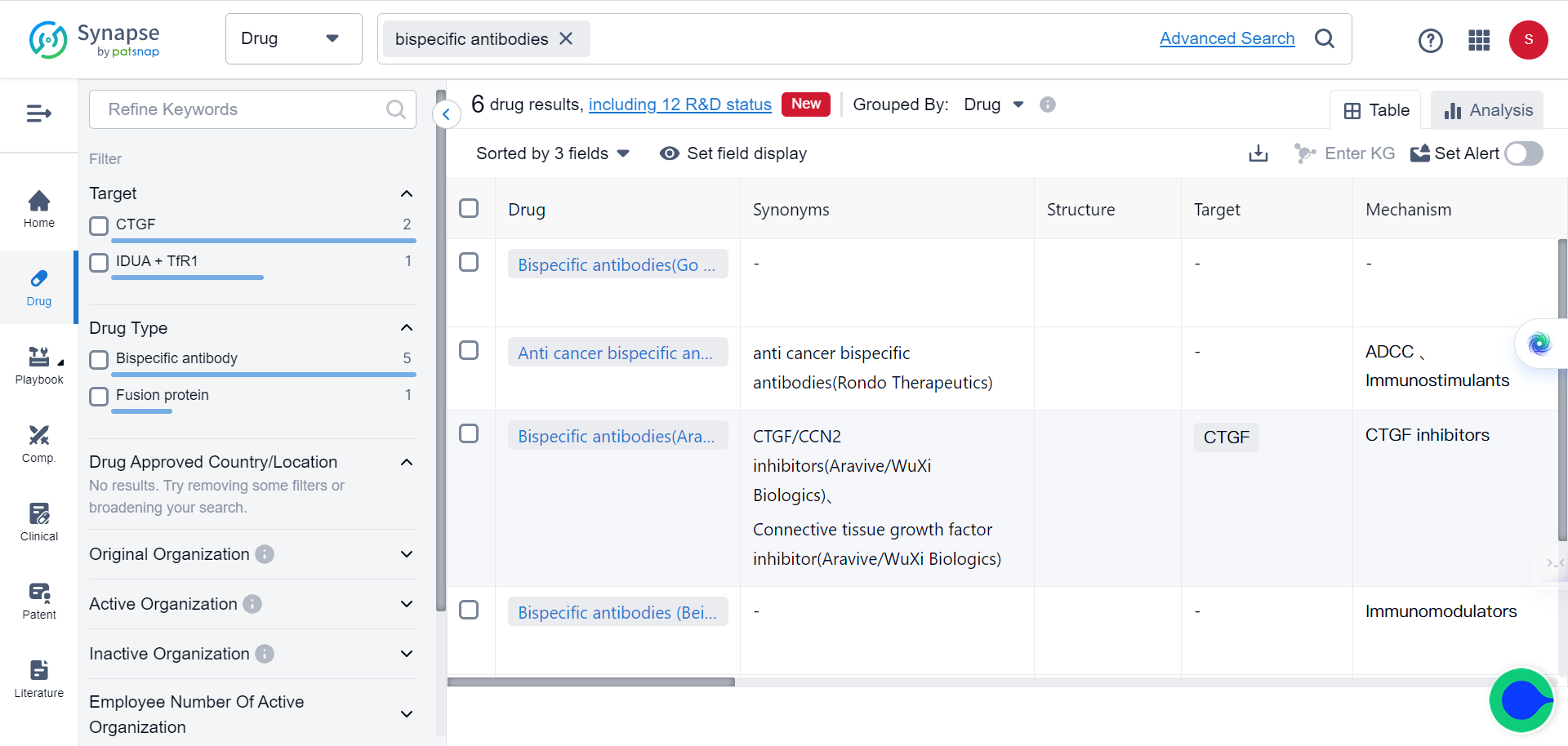
The Dual Boom of Bispecific Antibodies: Market and Drug Discovery Pipeline
Bispecific antibodies (BsAbs) are recombinant molecules that contain two different antigens or epitopes identifying binding domains. They have gained broad potential in preclinical and clinical investigations in a variety of tumor types following regulatory approval of newly developed technologies involving bispecific and multispecific antibodies.

Booming market
In fact, over 85% of bispecific antibodies in clinical trials are being designed as cancer treatments. In Synapse database, as of 2022, six bispecific antibodies have been approved by the FDA and/or EMA for cancer immunotherapy.
The first approval, Catumaxomab, was later withdrawn from the EU market in 2017. Three other bispecific antibodies gained approval in 2022. Additionally, marketing applications for five bispecific antibodies in late-stage trials may be submitted by the end of 2023.
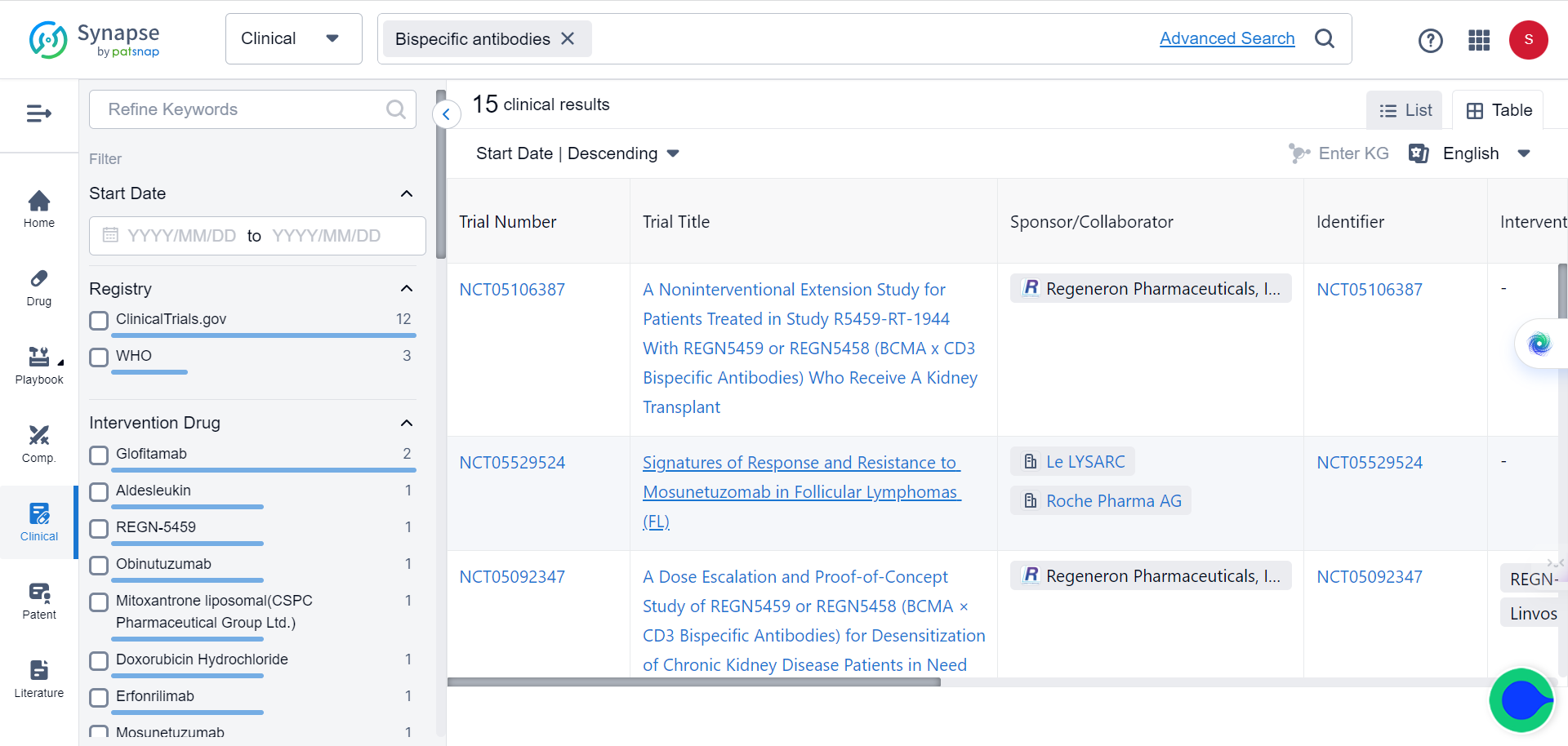
Currently over 600 bispecific antibodies are under investigation, as the sector is projected to grow to more than $30 billion over the next five years. Major pharmaceutical companies are increasingly interested in exploring and investing in this promising immunotherapy approach.
Diverse architectures & actions
Several architectures have been developed for BsAbs, each with different structures and mechanisms of action. IgG-like BsAbs are the most common and featured with an IgG-like structure with two different antigen-binding domains. Asymmetric IgG-like BsAbs possess one heavy chain and one light chain from each antigen-binding domain, while symmetric IgG-like BsAbs have two heavy chains and two light chains from each antigen-binding domain. Some examples of IgG-like BsAbs include blinatumomab and catumaxomab.

Diabodies, on the other hand, are small BsAbs that lack the Fc region and have two antigen-binding domains connected by a short linker. Their small size allows them to penetrate solid tumors more effectively than larger antibodies. Notable members from the diabodies family includes MT110 and RG7386.
A third type of BsAbs, known as dual-affinity retargeting (DART) molecules, are bispecific single-chain antibodies that have two different antigen-binding domains connected by a flexible linker. Due to their smaller size, similar to diabodies, as well as the high stability, they could be produced in large quantities and have a long shelf life. Molecules such as CD3xCD19 DART and CD3xCD123 DART fall into this category.
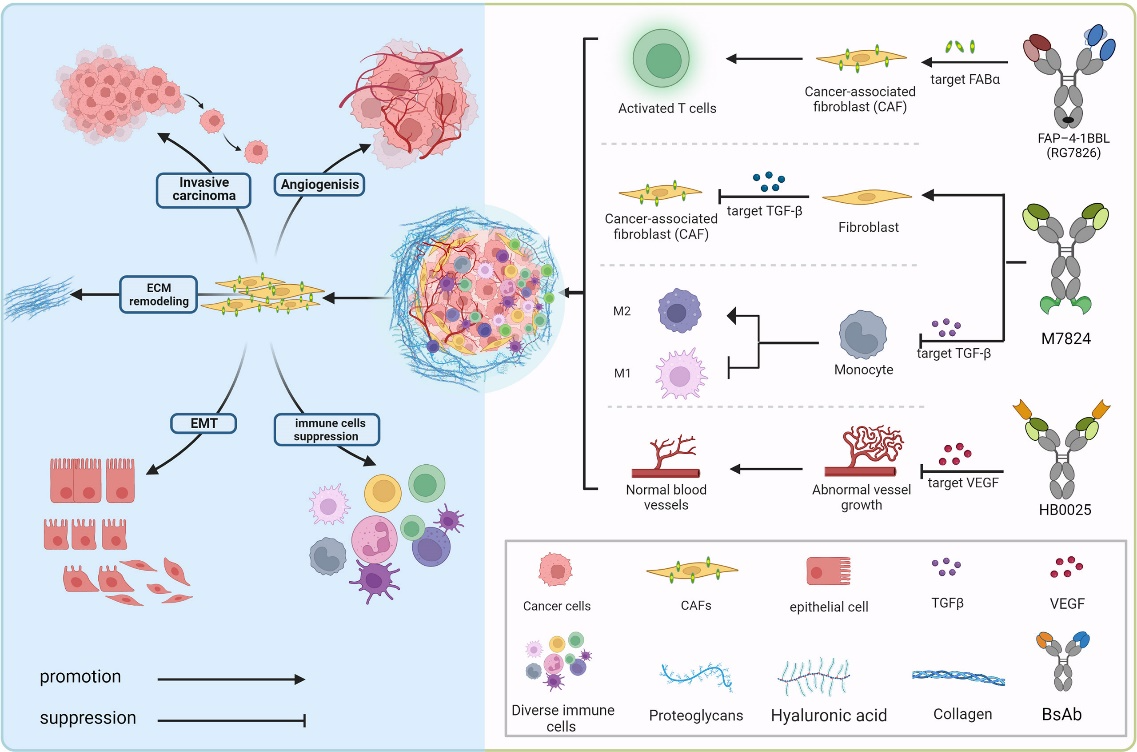
And there’s the BiTEs, which are bispecific T-cell engagers with one binding domain for a tumor antigen and another for the T-cell receptor. Their signature action is bringing T cells into close proximity with tumor cells, leading to T-cell activation and tumor cell killing. BiTEs have shown promising results in hematological malignancies and are being investigated in solid tumors.
The two binding domains offer BsAbs unique advantages otherwise impossible. A particular kind of BsAbs, checkpoint inhibitors, are raising high hopes among scientists, who believe that they have the potential to revolutionize anticancer treatment. The checkpoint inhibitor works by a smart strategy: blocking immune checkpoints through binding to both a tumor antigen and an immune checkpoint molecule, such as PD-1 or CTLA-4, thus enhancing T-cell activation.
With the development of these innovative BsAbs, cancer immunotherapy is entering a new era with the potential to improve treatment outcomes and patients’ quality of life.
High-throughput discovery pipeline
Seen by many as a smart arsenal for cancer treatment, the family of BsAbs is likely to expand even more quickly in near future, thanks to a high-throughput discovery pipeline.
In a recent study, researchers from the University of California, Irvine, and Amberstone Biosciences report to have developed a high-throughput, single-cell-based functional screening pipeline for the discovery of BsAbs.
The current discovery process for BsAbs is slow, complex, and expensive, which limits their broad clinical availability. By contrast, the newly-developed pipeline comprises molecular and cell engineering for efficient generation of BsAb library cells, followed by functional interrogation at the single-cell level to identify and sort positive clones and downstream sequence identification and functionality characterization. The technique allows the researchers to evaluate a large number of individual BsAb variants from an unbiased library.
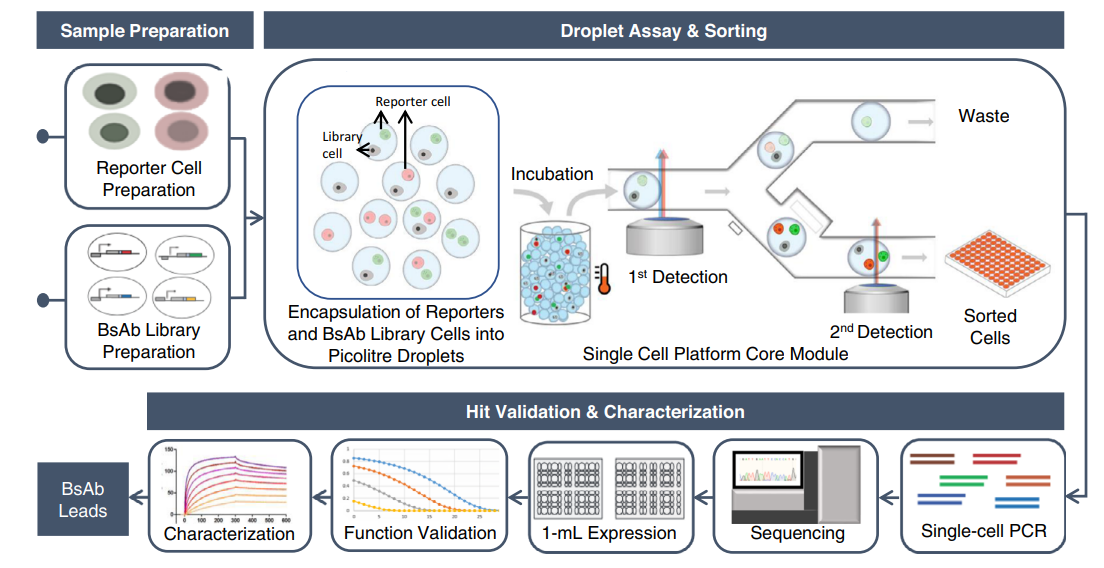
Using a CD19xCD3 bispecific T cell engager (BiTE) as a model, the single-cell platform demonstrated a high-throughput screening efficiency of up to one and a half million variant library cells per run and can isolate rare functional clones at a low abundance of 0.008%. The researchers identified 98 unique clones, including extremely rare ones (~ 0.001% abundance), from a complex CD19xCD3 BiTE-expressing cell library with approximately 22,300 unique variants comprising combinatorially varied scFvs, connecting linkers, and VL/VH orientations. They also discovered BiTEs that exhibit novel properties and insights to design variable preferences for functionality.
The single-cell platform employs a droplet microfluidic-based system to compartmentalize cells with co-encapsulated cell reporters. Functional BsAb clones activate cell reporters to produce fluorescence, allowing "positive" droplets to be detected and sorted from a heterogeneous population. This technique has the potential to increase the discovery efficiency of new immunotherapeutics while enabling researchers to identify generalizable design principles based on an in-depth understanding of the inter-relationships between sequence, structure, and function.

This latest development has the potential to transform the discovery of BsAbs, making the process more efficient, less costly, and more broadly available to patients suffering from a range of diseases, including cancer and autoimmunity.

References:
1.Wei J, Yang Y, Wang G and Liu M (2022) Current landscape and future directions of bispecific antibodies in cancer immunotherapy. Front. Immunol. 13:1035276.
2.Segaliny, A.I., Jayaraman, J., Chen, X. et al. A high throughput bispecific antibody discovery pipeline. Commun Biol 6, 380 (2023).