The GLP-1R plus strategy for curbing the obesity epidemic
Obesity has emerged as one of the gravest public health challenges of the 21st century. Data from the World Health Organization (WHO) shows that obesity has almost tripled globally since 1975, impacting over 650 million adults and 340 million children and adolescents in 2016. As of 2019, an estimated 38.2 million children under the age of 5 were considered overweight or obese.
Beyond GLP-1RA
This epidemic has created substantial demand for weight control medications. A new prescription approved by the U.S. Food and Drug Administration (FDA) in 2021, known as Wegovy or semaglutide, aims to help individuals with excess weight lose up to 15% of their body weight. The medication belongs to a class of drugs called glucagon-like peptide-1 receptor agonists (GLP-1 RAs), which mimic a natural hormone regulating appetite and blood sugar levels. However, these new therapies have drawbacks. Many patients cannot tolerate the side effects, and it’s estimated that within a year, 80 to 90% of individuals who start on these drugs discontinue their usage.
As a result, scientists are increasingly attempting to combine GLP-1 RAs with other hormone-based medications or engineer them as dual or triple receptor agonists to enhance their efficacy in treating obesity while mitigating side effects. This GLP-1R plus strategy, combining GLP-1 RAs with other hormone-based medications that target different pathways, such as amylin or GIP, may result in additive or synergistic effects that enhance weight loss and improve metabolic outcomes. Similarly, engineering GLP-1 RAs as dual or triple receptor agonists that target additional receptors beyond the GLP-1 receptor may provide greater efficacy in treating obesity by targeting multiple pathways simultaneously.
A search in Synapse database demonstrates that there are currently 128 drugs for treating obesity and overweight, with 14 different combinations of targets that contain GLP-1R.
Advent of triple agonists
Some of the combinations have drawn particular interests from researchers. For example, LY3437943, a novel triple agonist peptide discovered by Eli Lilly that targets the glucagon receptor (GCGR), glucose-dependent insulinotropic polypeptide receptor (GIPR), and GLP-1R (GCGR+GIPR+GLP-1R from the list above), can effectively reduce body weight and improve glycemic control in obese mice. LY3437943 may cause greater weight loss than Tirzepatide, a dual GIPR and GLP-1R agonist that has shown improved weight loss compared to selective GLP-1R agonists in patients with type 2 diabetes. In vitro studies have shown that LY3437943 has balanced GCGR and GLP-1R activity but more GIPR activity.
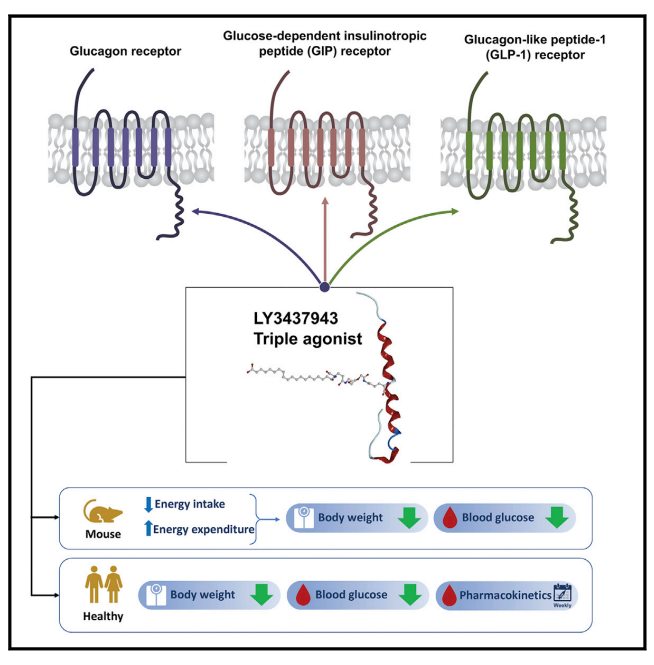
In obese mice, administration of LY3437943 resulted in decreased body weight and improved glycemic control. The weight loss was augmented by the addition of GCGR-mediated increases in energy expenditure to GIPR- and GLP-1R-driven calorie intake reduction.
In a phase 1 clinical trial, LY3437943 showed a safety and tolerability profile similar to other incretins. The pharmacokinetic profile also supported once-weekly dosing, and a reduction in body weight persisted up to day 43 after a single dose.
LY3437943 is certainly not the only drug with a trio of targets that includes GLP-1R, as can be seen from the earlier list, which is by no means exhaustive. With GLP-1R attracting more researchers and companies looking to curb the obesity epidemic, new drug candidates mushroom. Among them is the peptide triple agonist GEP44, which targets neuropeptide Y1 (Y1-R), and neuropeptide Y2 (Y2-R) receptors, along with GLP-1R. Synapse database indicates that 3 patents have been filed for this peptide:
GEP44 elicits Y1-R antagonist-controlled, GLP-1R-dependent stimulation of insulin secretion in both rat and human pancreatic islets, thus revealing the counteracting effects of Y1-R and GLP-1R agonism. It also promotes insulin-independent Y1-R-mediated glucose uptake in muscle tissue ex vivo and more profound reductions in food intake and body weight than liraglutide when administered to diet-induced obese rats. Additionally, the researchers found that GEP44 exhibits biased agonism at the Y2-R, as documented by its inability to induce Y2-R-mediated increases in intracellular Ca2+ or ERK1/2 phosphorylation.
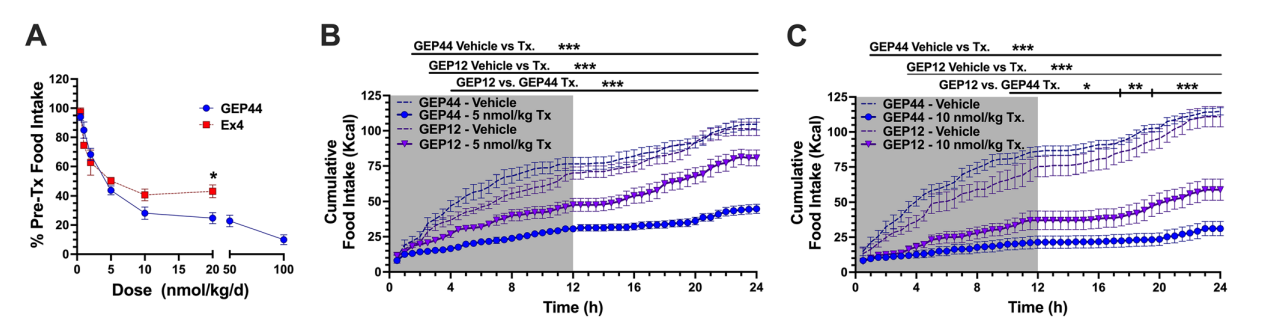
During the tests, rats and shrews administered with GEP44 showed no signs of nausea or vomiting, possibly due to the activation of multiple receptors canceling out the intracellular signaling pathway responsible for those symptoms.
The new generation
Nowadays, researchers are making remarkable progress in finding the next generation of triple agonists that could potentially offer greater weight loss benefits with fewer side effects. A case in point is the newly discovered unimolecular GLP-1R/GIPR/GCGR triple agonist. The Novo Nordisk prospect has been shown to normalize body weight in obese mice.
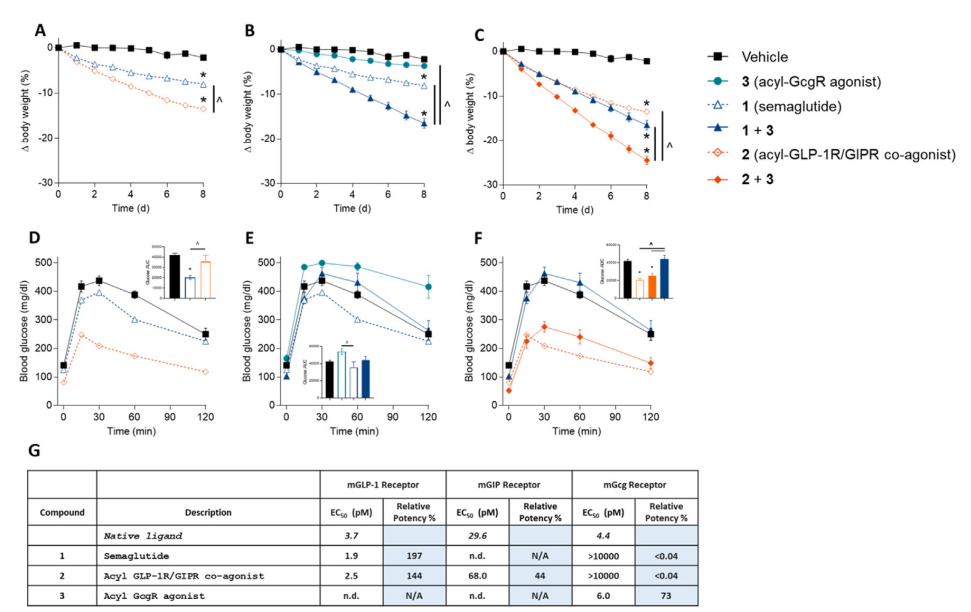
Contrary to GLP-1R mono-agonist, whose efficacy is limited by dose-dependent gastrointestinal events, the new triple agonists can simultaneously increase energy expenditure, reduce food intake, and improve glucose control by engaging all three receptors at once.
The researchers optimized the potency ratio between the three receptors and used a protraction strategy that allows for once-weekly dosing, then tested the drugs in diet-induced obese mice. The results were promising: the triple agonists were more effective at reducing body weight and increasing energy expenditure compared to GLP-1 receptor mono-agonists and GLP-1 receptor/GIP receptor co-agonists.
The GLP-1R plus strategy has enjoyed early success, although some of the treatments are still in the pre-clinical stage. Nevertheless, this approach brings new hope that we can finally address the growing concern of obesity worldwide.

References:
1.A. M. Jastreboff, R. F. Kushner, New Frontiers in Obesity Treatment: GLP-1 and Nascent Nutrient-Stimulated Hormone-Based Therapeutics, Annual Review of Medicine 2023. 74:125–39.
2.T. Coskun, S. Urva, et al., LY3437943, a novel triple glucagon, GIP, and GLP-1 receptor agonist for glycemic control and weight loss: From discovery to clinical proof of concept, 2022, Cell Metabolism 34, 1234–1247.
3.P. J. Knerr, S. A. Mowery et al., Next generation GLP-1/GIP/glucagon triple agonists normalize body weight in obese mice, Molecular Metabolism 63 (2022) 101533.
4.Chichura, K.S., Elfers, C.T., Salameh, T.S. et al. A peptide triple agonist of GLP-1, neuropeptide Y1, and neuropeptide Y2 receptors promotes glycemic control and weight loss. Scientific Reports 13, 9554 (2023). https://doi.org/10.1038/s41598-023-36178-1.