The FDA gives the go-ahead to Novartis Cosentyx® - a first biological treatment innovation for hidradenitis suppurativa sufferers in roughly ten years
The worldwide frontrunner in immuno-dermatology and rheumatology, Novartis, reported FDA's approval of Cosentyx® (secukinumab) for the management of moderate to extreme hidradenitis suppurativa in adult patients. Distinctly, Cosentyx is the one and only FDA-sanctioned purely human biologic that actively impedes the functioning of interleukin-17A (IL-17A), a pro-inflammatory cytokine suspected to have a role in the inflammation associated with HS.
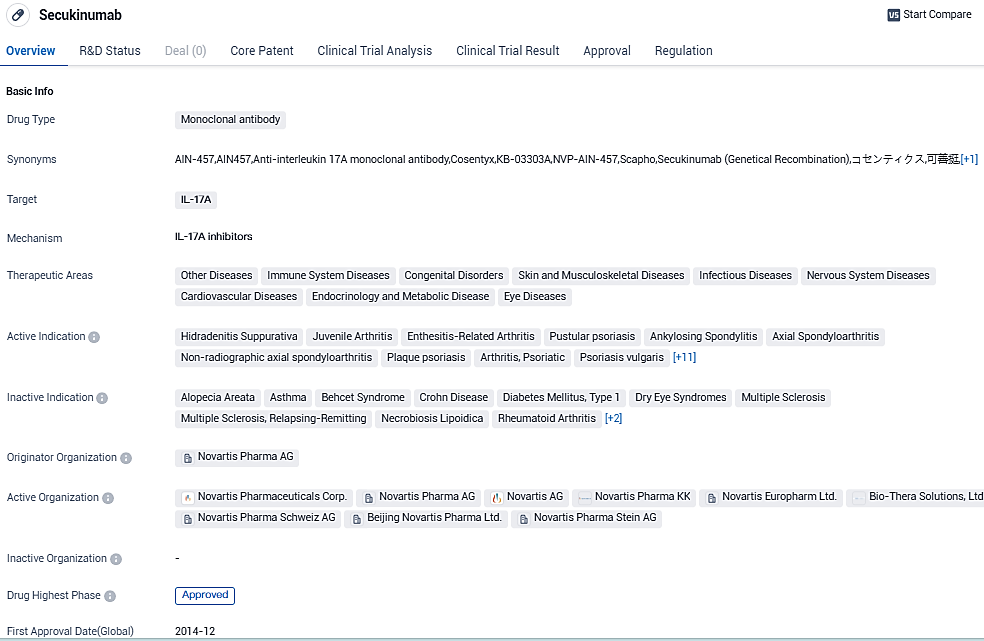
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
HS is an often painful, persistent skin condition that regularly results in lump-like boils, which may open into wounds and lead to permanent scarring, frequently affecting the most private regions of the body. On average, it might take up to a decade for individuals affected by HS to receive an accurate diagnosis, possibly leading to disease advancement and major disruptions to their everyday life quality. Only one biologic treatment for HS has been approved up to this point.
According to Dr. Alexa B. Kimball, the main investigator for the SUNSHINE and SUNRISE trials and Harvard Medical School Dermatology Professor, the daily burden of HS and the lengthy quest for symptom relief endured by many patients often results in agonizing, immovable physical and emotional scars. She further noted the new therapy approval as a significant achievement for countless patients who had previously faced narrow treatment options.
Donna Atherton, EdD, Founder and Chief Mission Officer of the International Association of Hidradenitis Suppurativa Network, describes HS as one of the most draining and tragic skin diseases, with flare pain severely hampering her ability to work or engage in social events. It profoundly affects her both physically and emotionally, leading to feelings of anxiety, stress, and solitude.
Novartis US president Victor Bultó indicated that Cosentyx could provide lasting and efficient relief from HS symptoms, giving individuals with HS the opportunity to live each day confidently. Cosentyx's sixth-indication approval highlights our unwavering commitment to revolutionize treatment for those affected by immune diseases, he added.
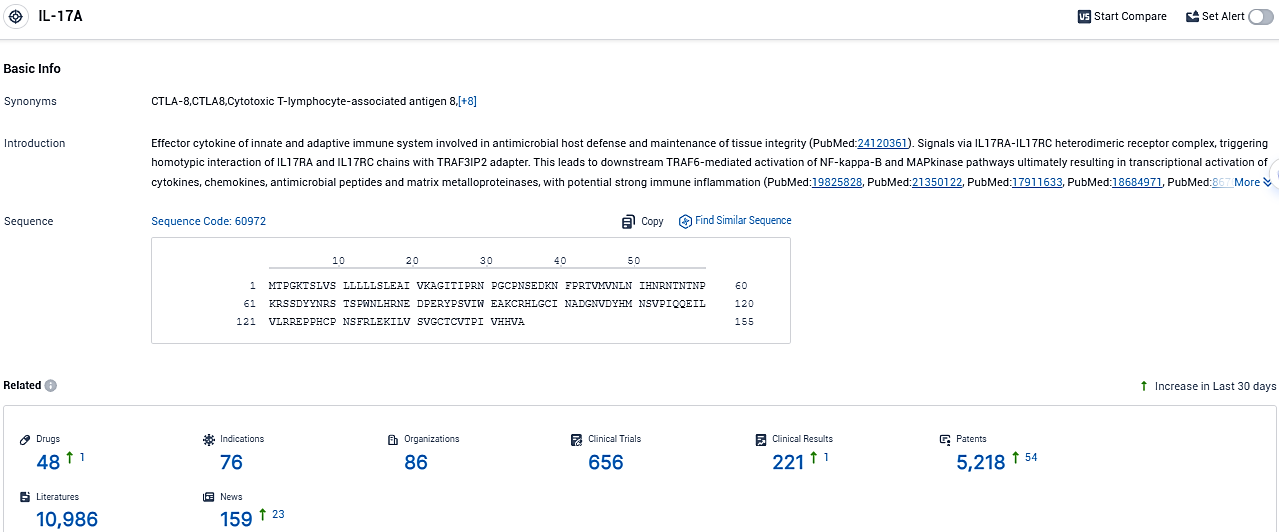
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 6, 2023, there are 48 investigational drugs for the IL-17A target, including 76 indications, 86 R&D institutions involved, with related clinical trials reaching 656, and as many as 10986 patents.
Cosentyx is the first and only fully human biologic that specifically targets and blocks interleukin-17A (IL-17A), an important cytokine involved in the inflammation of psoriatic arthritis, moderate to severe plaque psoriasis, ankylosing spondylitis and non-radiographic axial spondyloarthritis. Cosentyx is a proven medicine and has been studied clinically for more than 14 years. Cosentyx is approved in more than 100 countries, most recently gaining approval for JIA and HS in the US and Europe.