The FDA has greenlighted a replicable bioequivalent treatment for various inflammation-related disorders
The FDA has given its sanction for Wezlana (ustekinumab-auub), considering it a biosimilar and substitute for Stelara (ustekinumab) in treating several inflammatory conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
 "Biosimilar pharmaceuticals bring about further safe and efficient therapeutic options that might enhance accessibility for those needing treatment for inflammatory diseases," stated Nikolay Nikolov, M.D., the head of the Office of Immunology and Inflammation within the FDA’s Center for Drug Evaluation and Research. "The authorization could contribute significantly for patients managing their condition."
"Biosimilar pharmaceuticals bring about further safe and efficient therapeutic options that might enhance accessibility for those needing treatment for inflammatory diseases," stated Nikolay Nikolov, M.D., the head of the Office of Immunology and Inflammation within the FDA’s Center for Drug Evaluation and Research. "The authorization could contribute significantly for patients managing their condition."
Biological substances encompass drugs treated for a wide range of severe diseases and chronic health problems. A biosimilar is a biological product that shows a high similarity, and possesses no evident clinical dissimilarities, to an already FDA approved biological product. An interchangeable biosimilar meets other legal requirements, making it a viable substitute for the reference product, even without the prescriber's consultation.
This replacement can happen within the pharmacy, and varies based on state pharmacy laws, a practice often termed "pharmacy-level substitution"—analogous to how generic drugs replace brand name drugs.
All biological products only receive approval after fulfilling the stringent approval standards of the FDA. Therefore, health care providers and patients can anticipate the same safety and effectiveness from a biosimilar and an interchangeable biosimilar, similar to a reference product. Biosimilar and interchangeable biosimilar items could cost lesser than the brand-name medication.
The FDA's ratification of Wezlana relies on a thorough examination of scientific proof indicating its high similarity to Stelara, and that no clinical meaningful variations exist between both products in terms of safety, purity, and potency. The evidence further confirmed that Wezlana fulfilled other statutory preconditions to be interchangeably used with Stelara at the pharmacy level.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
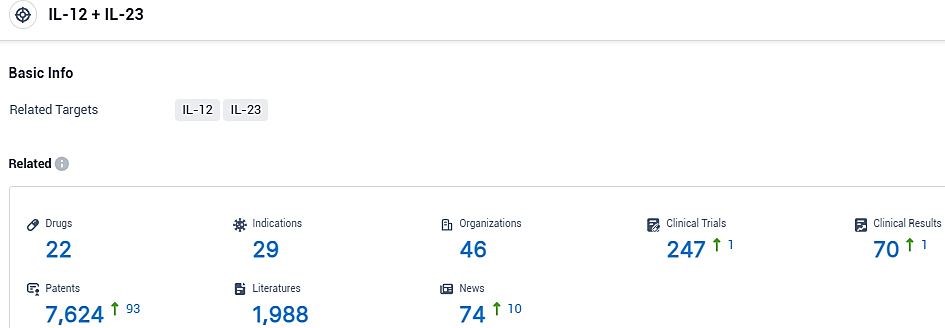
According to the data provided by the Synapse Database, As of November 6, 2023, there are 22 investigational drugs for the IL-12 and IL-23 target, including 29 indications, 46 R&D institutions involved, with related clinical trials reaching 247, and as many as 7624 patents.
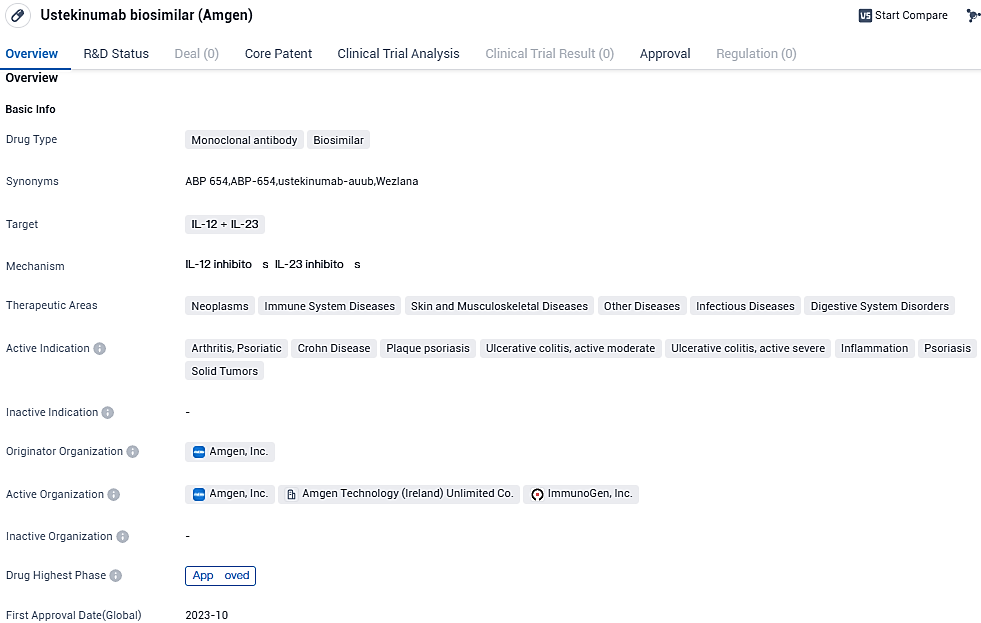
Ustekinumab biosimilar, developed by Amgen, is a monoclonal antibody drug that targets IL-12 and IL-23. It has shown promise in treating various diseases across different therapeutic areas, including neoplasms, immune system diseases, skin and musculoskeletal diseases, other diseases, infectious diseases, and digestive system disorders. The drug's active indications encompass arthritis, psoriatic, Crohn's disease, plaque psoriasis, ulcerative colitis, inflammation, psoriasis, and solid tumors.