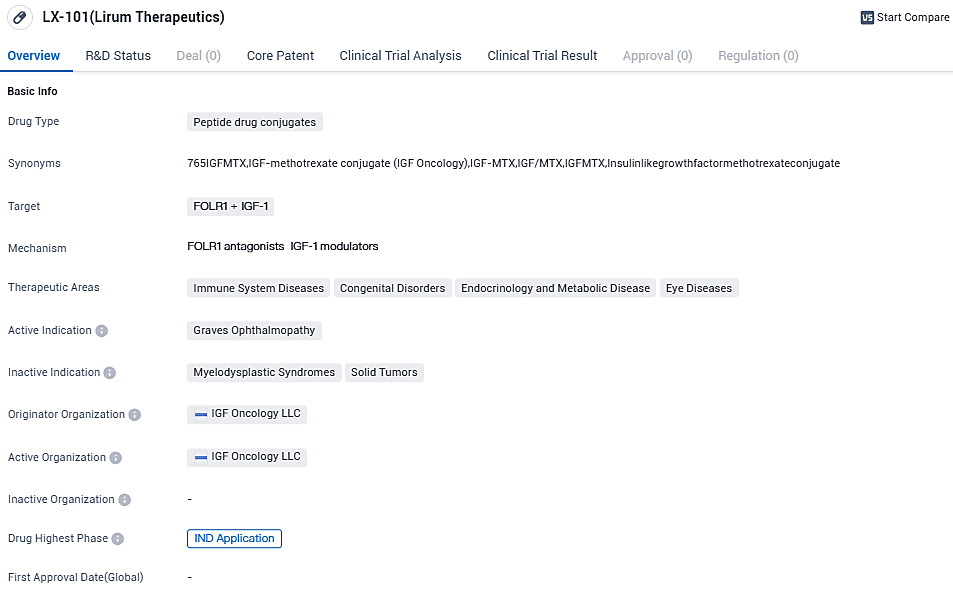
Lirum Therapeutics reveals promising preliminary results of targeted clinical treatment LX-101's notable efficacy against children's sarcomas
At the 2023 Connective Tissue Oncology Society gathering in Dublin, Lirum Therapeutics, Inc., a pioneering biopharmaceutical organization conducting clinical trials for the treatment of crippling illnesses, publicly revealed encouraging research outcomes.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
LX-101, an innovative medicine currently at the clinical-stage, is a specialized therapy that specifically targets the insulin-like growth factor 1 receptor (IGF-1R). It has shown considerable effectiveness in fighting against specific cell constitutions that display a prominent IGF-1R pathway, which includes pediatric sarcomas. This category encompasses cancers that carry well-documented genetic modifications interfering with the IGF-1R pathway or cancers that have a high IGF-1R manifestation such as Ewing’s or connected sarcomas, rhabdomyosarcoma, and osteosarcoma.
Building on the encouraging evidence and data already accumulated, Lirum intends to conduct additional clinical trials concerning LX-101, focusing on both pediatric and adult cancer patients who have a strong link to the IGF-1/IGF-1R pathway. Concurrently, Lirum plans to expand LX-101 treatments to certain autoimmune disorders, including thyroid eye disease, where the IGF-1R has been medically and commercially confirmed.
Lirum's leading candidate product LX-101 is an innovative clinical-phase precision-engineered remedy dedicated targeting IGF-1R. It's proven the IGF-1/IGF-1R pathway plays a significant role in numerous cancers and autoimmune diseases, including thyroid eye disease. Lirum stands firm in its belief that it's a logical and scientifically based approach to target it for cancer and autoimmune disease treatment.
LX-101 is structured with an exclusive variation of the IGF-1 ligand, bound to perilous payload, methotrexate, displaying a unique and different action mechanism compared to other past and present IGF-1R targeted methods.
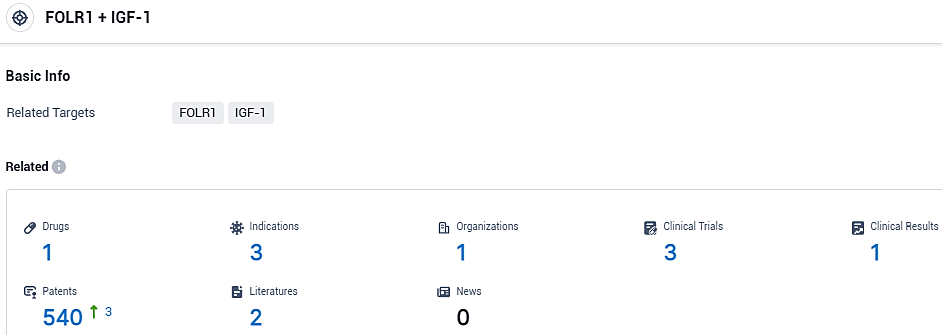
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 6, 2023, there are 1 investigational drugs for the FOLR1 and IGF-1 target, including 3 indications, 1 R&D institutions involved, with related clinical trials reaching 3, and as many as 540 patents.
LX-101 has been evaluated in Phase 1 clinical trials of patients with advanced, pretreated solid and hematologic cancers. It was found to be well-tolerated and demonstrated single agent activity. No dose limiting toxicity or maximum tolerated dose were reached, and Lirum intends to explore further dose escalation and schedule optimization as well as focus on those indications with strong ties to the IGF-1/IGF-1R pathway.