The FDA Grants Approval for Biosyngen’s BRG01 to Begin Phase II Clinical Trial
Biosyngen is excited to share that the U.S. Food and Drug Administration (FDA) has given the green light for its BRG01, an EBV-specific CAR-T cell therapy, to advance to a crucial Phase II clinical trial. This pioneering treatment is the inaugural cell therapy to commence Phase II trials in both the United States and China for patients suffering from relapsed/metastatic EBV-positive nasopharyngeal carcinoma, signifying a significant advancement in the treatment of solid tumors.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The Center for Drug Evaluation (CDE) under the National Medicinal Product Administration (NMPA) in China has previously given the green light for the pivotal Phase II study of BRG01. Enrollment of patients for the Phase I trial in China and the United States commenced at the end of January this year, and all subjects have since completed their BRG01 infusions. The Phase I trial has successfully wrapped up its dose-limiting toxicity (DLT) observation and efficacy evaluation for nine advanced-stage nasopharyngeal carcinoma patients, all of whom had received at least one prior immune checkpoint inhibitor treatment, encompassing PD-1 antibodies.
Initial results suggest that BRG01 possesses outstanding safety and early signs of efficacy. The entire cohort comprises advanced cancer patients who have not responded to standard treatments, including checkpoint inhibitors. BRG01 has shown good tolerability and expansion in patients without any dose-limiting toxicity. Increased disease control and tumor reduction were noted with higher doses. In the high-dose group, 75% of patients exhibited tumor lesion necrosis and metabolic reduction, as assessed via PET-CT scans.
Biosyngen is focused on accelerating the clinical progression and commercialization of BRG01, providing a new beacon of hope for nasopharyngeal cancer patients globally. This approval by the FDA highlights BRG01’s potential in both tumor and antiviral treatments and acknowledges Biosyngen’s innovative prowess and R&D strengths in cellular immunotherapy.
Biosyngen has emerged as a premier biotech entity with a suite of cell therapies, such as CAR-T, TCRT, and TIL, targeting various solid tumors and blood cancers. The company's diverse products have obtained approvals to launch Phase I/II trials in both the U.S. and China, targeting different solid tumors like lung and liver cancer.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
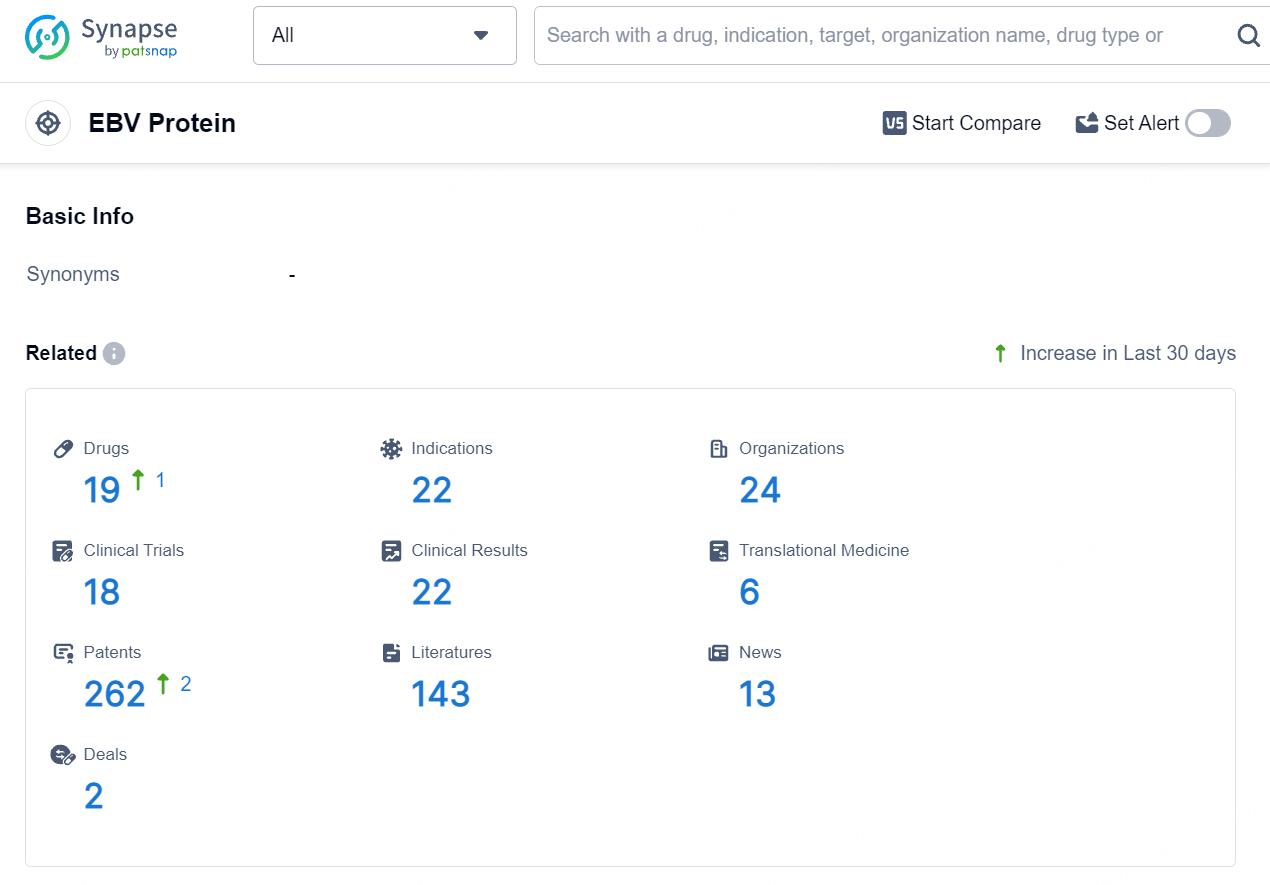
According to the data provided by the Synapse Database, As of August 15, 2024, there are 19 investigational drugs for the EBV Protein target, including 22 indications, 24 R&D institutions involved, with related clinical trials reaching 18, and as many as 262 patents.
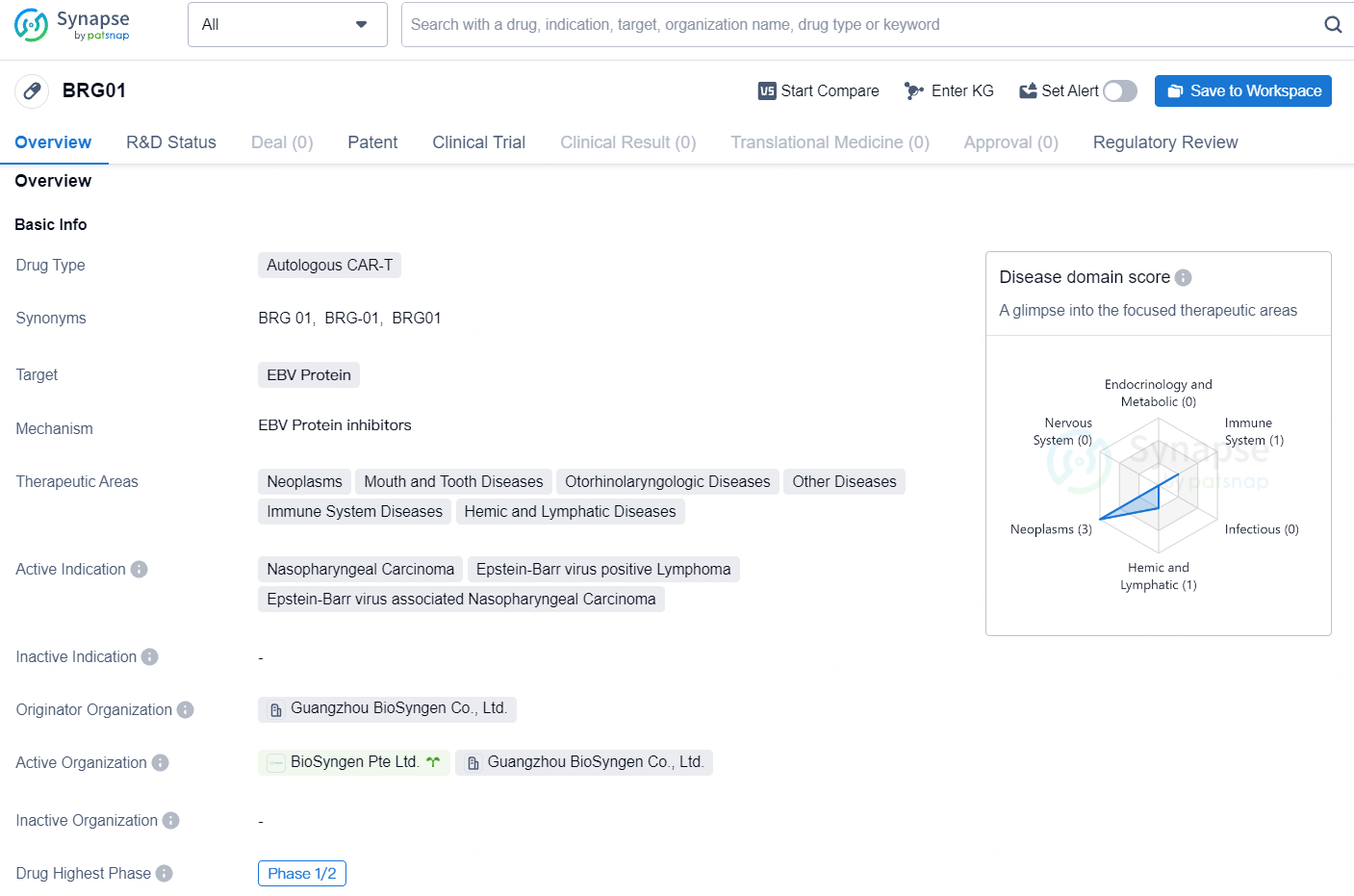
BRG01 is an autologous CAR-T drug that targets the EBV Protein and is being developed by Guangzhou BioSyngen Co., Ltd. The drug falls under the therapeutic areas of Neoplasms, Mouth and Tooth Diseases, Otorhinolaryngologic Diseases, Other Diseases, Immune System Diseases, and Hemic and Lymphatic Diseases. The active indications for [BRG01] include Nasopharyngeal Carcinoma, Epstein-Barr virus positive Lymphoma, and Epstein-Barr virus associated Nasopharyngeal Carcinoma.