First Patient Dosed in Phase 2 Anti-TIGIT Therapy Trial for Advanced Liver Cancer
Shanghai Henlius Biotech, Inc. (2696.HK) reported that the initial patient has been administered a dose in a phase 2 clinical trial (NCT06349980) involving HLX53, an anti-TIGIT Fc fusion protein, in combination with HANSIZHUANG (serplulimab, HLX10) and HANBEITAI (bevacizumab, HLX04) for the first-line treatment of patients with locally advanced or metastatic hepatocellular carcinoma (HCC).
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Liver cancer ranks among the most widespread malignancies globally. As reported by GLOBALCAN 2022, approximately 870,000 new cases and about 760,000 deaths were recorded worldwide. In China, primary liver cancer (PLC) is the fifth most common cause and the second leading cause of cancer-related mortality, with around 370,000 new cases and 320,000 deaths in 2022.
Hepatocellular carcinoma (HCC) is the most dominant pathological type of PLC, constituting between 75% and 85% of liver cancer cases. Due to its stealthy onset, absence of early-stage symptoms, and rapid development, PLC is often diagnosed at a locally advanced stage or after distant metastasis has occurred. Consequently, treatment becomes very challenging and the prognosis is generally poor. The 5-year survival rate for PLC is merely around 18%.
For those with advanced liver cancer, the primary treatment involves targeted therapy and immunotherapy. The standard approach, which combines immune inhibition with anti-angiogenic treatments, has demonstrated significant anti-tumour effects and survival benefits. Nevertheless, some patients do not benefit from the standard treatment, experiencing disease recurrence or progression. There remains a pressing clinical need to broaden the patient population benefiting from advanced liver cancer treatments and to enhance the effectiveness of immunotherapy.
- cell immunoglobulin and ITIM domains (TIGIT) has become a notable inhibitory checkpoint receptor, predominantly found on natural killer (NK) cells, activated CD8+ T cells, CD4+ T cells, and regulatory T cells. TIGIT interacts with the ligand CD155 (poliovirus receptor, PVR), typically expressed on antigen-presenting cells (APC) or tumour cell surfaces, to suppress T cell and NK cell activity.
- As an immune checkpoint protein, TIGIT can inhibit both innate and adaptive immune responses through various mechanisms, functioning similarly to PD-1/PD-L1 by acting as "brakes" to halt T cells from attacking tumours. Research has demonstrated that TIGIT inhibitors are effective against various cancers such as lung cancer, gastric cancer, melanoma, and multiple myeloma. Additionally, TIGIT has shown synergistic effects with the PD-1 pathway in preclinical studies, suggesting that concurrent inhibition of TIGIT and PD-1/PD-L1 signaling pathways is more effective than inhibiting either pathway alone, thereby enhancing anti-tumour activity.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
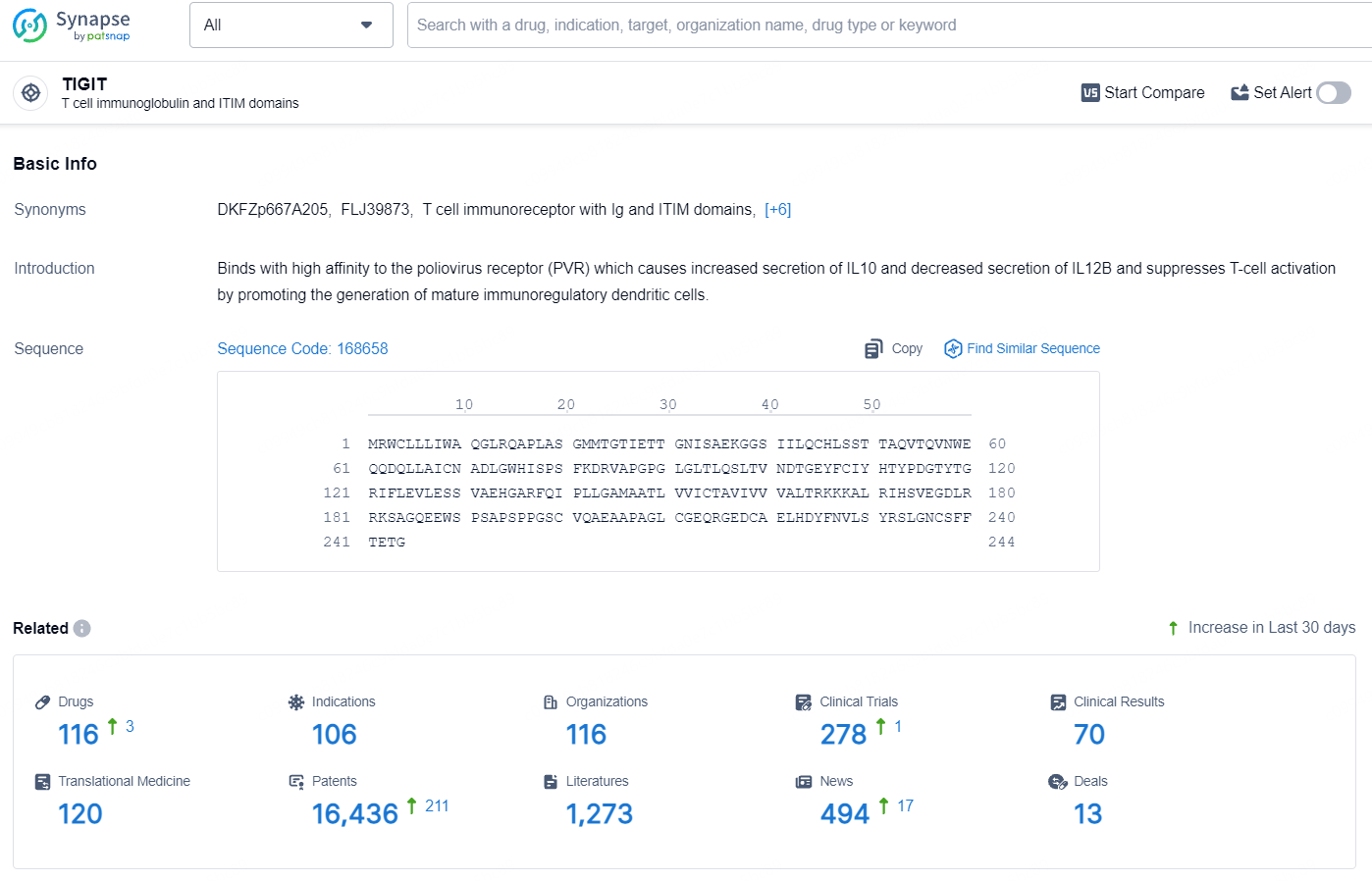
According to the data provided by the Synapse Database, As of August 14, 2024, there are 116 investigational drugs for the TIGIT target, including 106 indications, 116 R&D institutions involved, with related clinical trials reaching 278, and as many as 16436 patents.
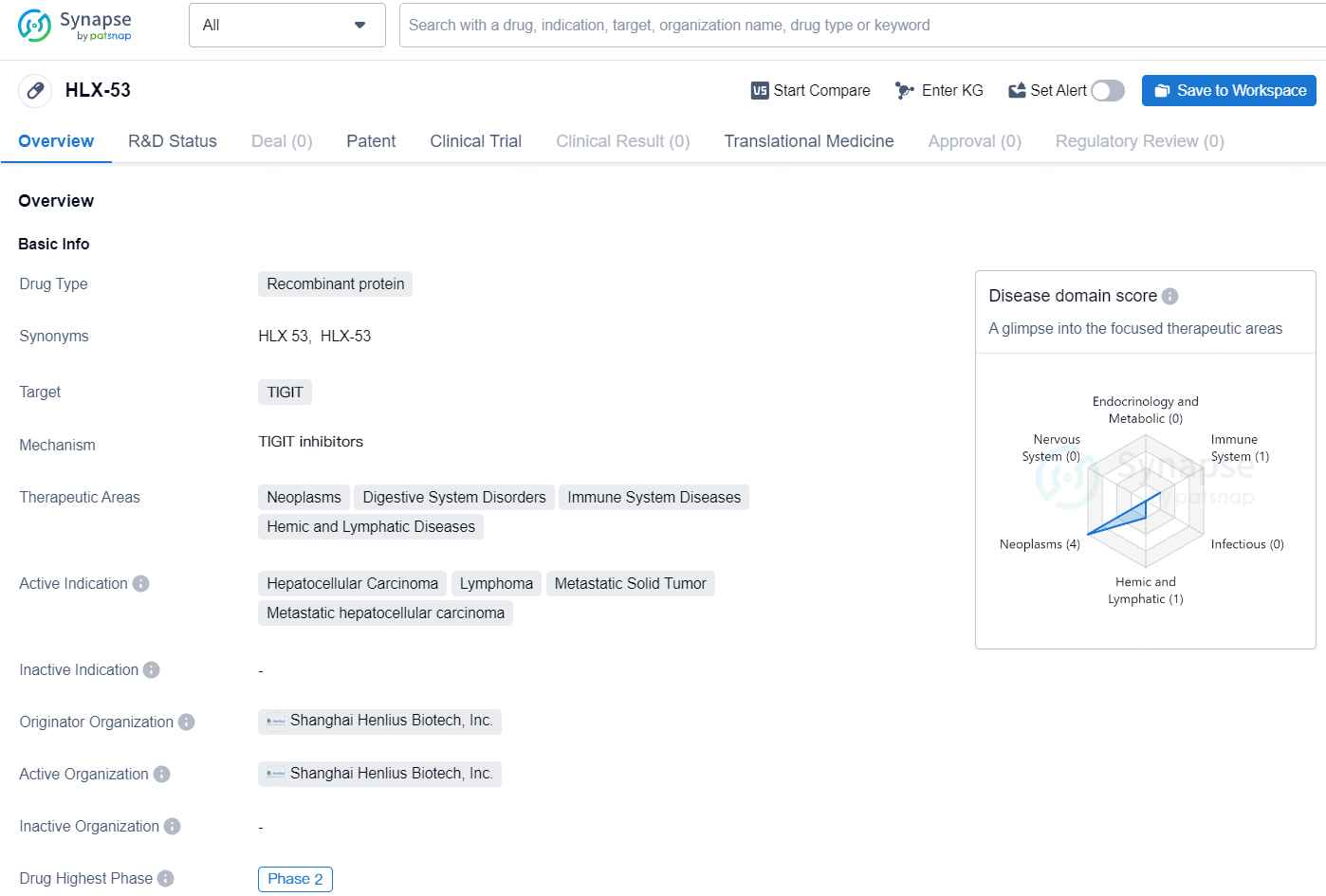
HLX53 is an anti-TIGIT Fc fusion protein independently developed by Henlius, consisting of variable domain of heavy chain of heavy-chain antibody (VHH) and wildtype IgG1 Fc. Pre-clinical studies have demonstrated that HLX53 exhibits excellent tumour inhibition with good safety. In addition, Henlius has initiated a phase 1 study to evaluate the safety, tolerability, pharmacokinetics characteristics and preliminary efficacy of HLX53 in patients with advanced/metastatic solid tumors.