VISEN Pharmaceuticals' Phase 3 Trial of Palopegteriparatide Successfully Meets Major Goals in Treating Hypoparathyroidism
VISEN Pharmaceuticals, a pioneering firm in the biopharmaceutical arena concentrating on endocrine disorders, has today shared top results from the 26-week randomized, double-blind, placebo-controlled segment of the Phase 3 PaTHway China Trial of Palopegteriparatide (TransCon PTH) in patients with long-term hypoparathyroidism. The analysis revealed a significantly greater percentage of patients receiving palopegteriparatide met the primary multi-component endpoint in comparison to those given a placebo.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 On August 12, 2024, in Shanghai, China, VISEN Pharmaceuticals, a progressive biopharmaceutical enterprise specializing in endocrine disorders, unveiled the top-level findings from the 26-week randomized, double-blind, placebo-controlled Phase 3 PaTHway China study of Palopegteriparatide (TransCon PTH) for adults with chronic hypoparathyroidism. The data revealed that a significantly larger percentage of patients administered palopegteriparatide met the main multi-component endpoint compared to those given a placebo.
On August 12, 2024, in Shanghai, China, VISEN Pharmaceuticals, a progressive biopharmaceutical enterprise specializing in endocrine disorders, unveiled the top-level findings from the 26-week randomized, double-blind, placebo-controlled Phase 3 PaTHway China study of Palopegteriparatide (TransCon PTH) for adults with chronic hypoparathyroidism. The data revealed that a significantly larger percentage of patients administered palopegteriparatide met the main multi-component endpoint compared to those given a placebo.
The primary multi-component endpoint, as described for the PaTHway China trial, was the attainment of normal serum calcium levels (8.3–10.6 mg/dL or 2.07-2.64 mmol/L), alongside independence from traditional therapy (requiring no active vitamin D and ≤600 mg/day of oral calcium), without an increase in the prescribed study medication within the four weeks leading up to the Week 26 visit. This endpoint was met by 77.6% of the patients treated with palopegteriparatide (45 out of 58), compared to none (0 out of 22) in the placebo cohort (p-value <0.0001). These results align with those previously reported by Ascendis Pharma for its Phase 3 trial of palopegteriparatide.
Professor XIA Weibo, the chief researcher of the trial and Director of the Department of Endocrinology at Peking Union Medical College Hospital, commented: “Hypoparathyroidism remains the only endocrine deficiency where hormone replacement is not yet a standard treatment. This trial demonstrates that parathyroid hormone (PTH) replacement therapy can effectively alleviate symptoms due to blood calcium fluctuations and help maintain normal serum calcium levels without exacerbating hypercalciuria.
Notably, TransCon PTH treatment normalized urine calcium levels. By offering physiologic PTH and diminishing the reliance on oral calcium supplements and active vitamin D for most patients, it may lower the long-term risks associated with conventional treatments, like soft-tissue calcifications and reduced kidney function. I believe that PTH replacement therapy could markedly enhance the overall health and life quality for those with hypoparathyroidism.”
In this study, 81 hypoparathyroidism patients were randomly assigned in a 3:1 ratio to either palopegteriparatide or placebo groups. Initially, all participants were on standard therapy. Both groups underwent an adjustment phase of traditional therapy and study treatment (palopegteriparatide or placebo) based on an algorithm focused on serum calcium levels, intending to achieve independence from conventional treatment.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
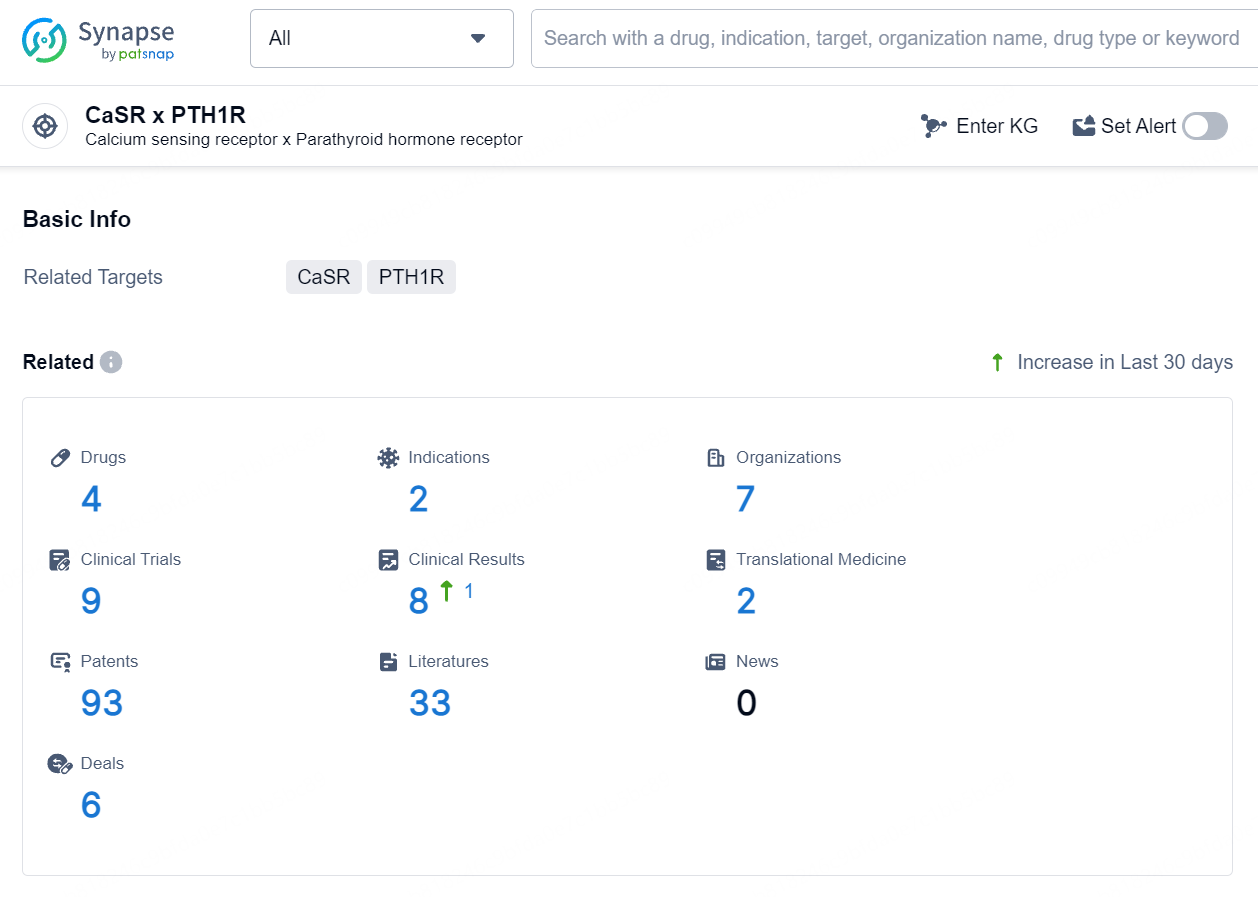
According to the data provided by the Synapse Database, As of August 14, 2024, there are 4 investigational drugs for the CaSR x PTH1R target, including 2 indications, 7 R&D institutions involved, with related clinical trials reaching 9, and as many as 93 patents.
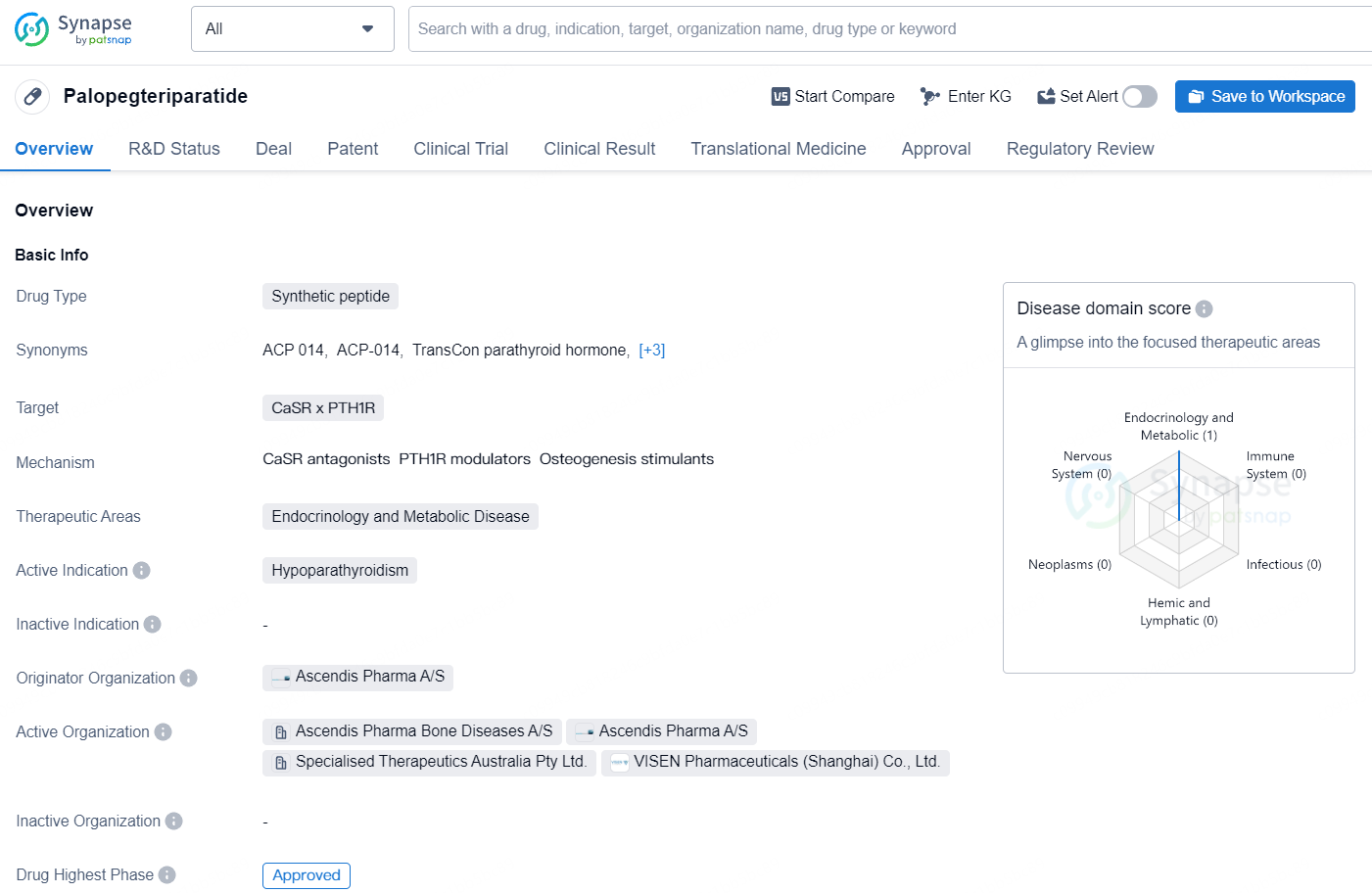
Palopegteriparatide is a synthetic peptide drug that targets the calcium-sensing receptor (CaSR) and the parathyroid hormone receptor 1 (PTH1R). It falls under the therapeutic areas of endocrinology and metabolic disease, and its active indication is for the treatment of hypoparathyroidism.