The FDA receives Lantern Pharma's IND, paving the way to Initiate Phase 1 trials for LP-284 in Non-Hodgkin’s Lymphomas
Artificial intelligence-focused Lantern Pharma Inc., who is employing their exclusive RADR® AI and machine learning system to innovate unique cancer remedies, unveiled the FDA's approval of their IND application for LP-284. The specific use of LP-284 is in the medical management of non-Hodgkin’s lymphoma that has either relapsed or become refractory, inclusive of mantle cell lymphoma and double hit lymphoma and other higher-grade B-cell lymphomas. Lantern anticipates initiating participant recruitment for the initial human Phase 1 trial involving LP-284 within the final quarter of 2023.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"It's become the second novel drug candidate of ours that the FDA has granted IND approval within the previous 100 days, further substantiating our approach of incorporating AI and machine learning to hasten the progress of our pipeline," declared Panna Sharma, the CEO and President of Lantern.
According to Sharma, RADR® was instrumental in decoding how LP-284 works, in assigning its priorities when it comes to treating different forms of cancer, and in generating machine learning-driven biomarker signs that may be crucial to patient selection in future clinical trial stages. "LP-284's progress so far is proof of our unwavering determination to utilize AI technology to revolutionize cancer treatment," she said.
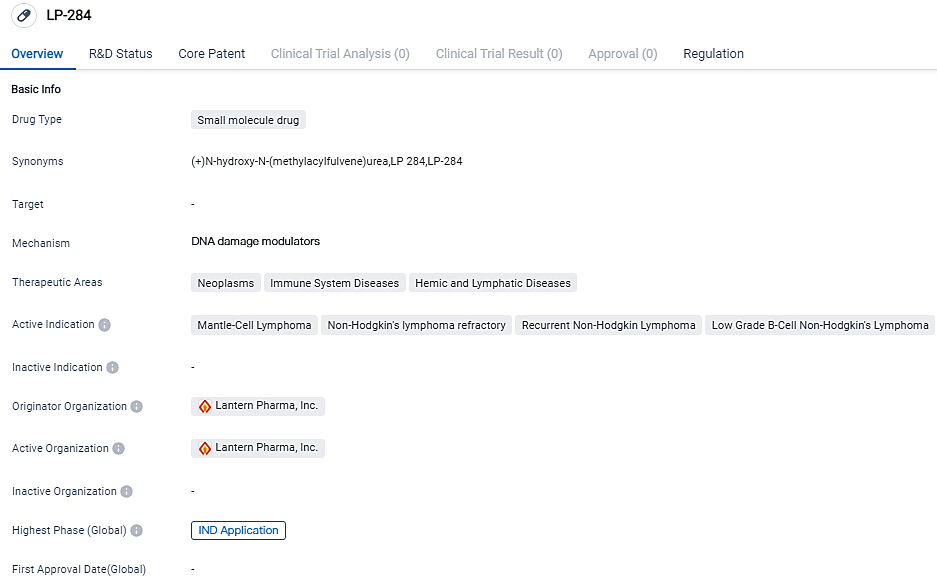
LP-284 is an innovative small molecule with a synthetically lethal mode of action that specifically targets cancer cells with DNA damage repair pathway mutations. Lantern’s LP-284 program has been accelerated and focused using AI insights and biological modelling facilitated by RADR®.
NHL, which is the top hematological malignancy in the US and a leading cause of cancer deaths worldwide, registers roughly 500,000 new cases globally each year. Despite advancements in NHL therapy combining targeted treatment methods, 20% to 40% of patients inflicted with certain subtypes relapse post-therapy. This includes almost all patients suffering from MCL, an aggressive NHL subtype. The potential annual market value of LP-284 in NHL treatment globally is estimated to be around $4 billion USD.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
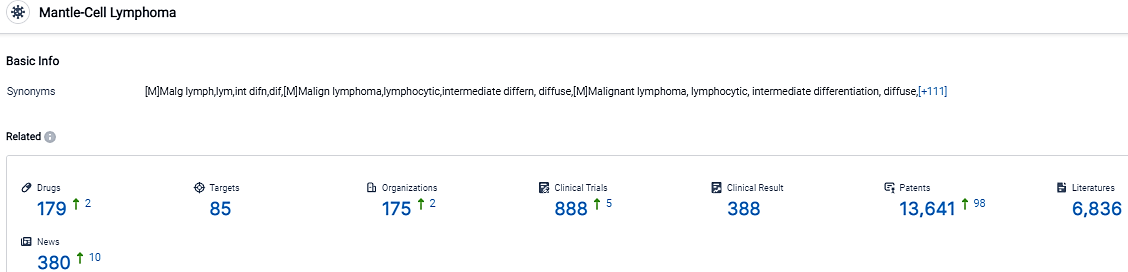
According to the data provided by the Synapse Database, As of September 22, 2023, there are 179 investigational drugs for the Mantle-Cell Lymphoma, including 85 targets,175 R&D institutions involved, with related clinical trials reaching 888,and as many as 13641 patents.
LP-284 developed for the treatment of neoplasms, immune system diseases, and hemic and lymphatic diseases. It is indicated for mantle-cell lymphoma, non-Hodgkin's lymphoma refractory, recurrent non-Hodgkin lymphoma, and low-grade B-cell non-Hodgkin's lymphoma. LP-284 is currently in the IND application phase, and its orphan drug designation highlights its potential to address rare diseases. Further research and development are needed to establish its effectiveness and safety.