Precise Therapy for Leukemia – IDH1 Inhibitors
The tricarboxylic acid (TCA) cycle, also known as the citric acid cycle or Krebs cycle, comprises of eight steps. It is the process of fully oxidizing acetyl coenzyme A to generate carbon dioxide, which is the third stage in catabolic metabolism. The third stage involves the oxidative decarboxylation of isocitric acid, forming alpha-ketoglutaric acid (α-KG). This is the first oxidation in the tricarboxylic acid cycle, catalyzed by isocitrate dehydrogenase (IDH), to produce reduced nicotinamide adenine dinucleotide phosphate (NADPH). The intermediate is oxalosuccinic acid.
IDH includes three subtypes -IDH1, IDH2, and IDH3. Depending on the subtype, they can be monomers, homodimers, and polymers. IDH1 exists as a homodimer, with each homodimer formed by the combination of two IDH1 monomers. IDH1 and IDH2 are isoenzymes with highly similar structures. IDH3 is a heterotetramer, containing 2 α subunits, 1 β subunit, and 1 γ subunit.
IDH1 is located in the cytoplasm and peroxisomes, while IDH2 and IDH3 are found in the mitochondria. IDH1 and IDH2 are nicotinamide adenine dinucleotide phosphate (NADP+) dependent metabolic enzymes that catalyze the conversion of isocitrate to alpha-ketoglutarate (α-KG), simultaneously reducing NADP+ to NADPH. NADPH is a key cellular reductant necessary for detoxification processes, controlling the mechanism of cellular defense against oxidative damage through the reduction of glutathione and thioredoxin, and the formation of activated hydrogen peroxide enzymes. Therefore, the deficiency of IDH1/2 in tumor cells can impair the detoxification mechanism, leading to DNA damage and genomic instability.
Mutations in IDH1 and IDH2 are closely associated with tumor onset and progression. IDH1 mutations are common in solid tumors, IDH2 mutations are common in hematological malignancies, but IDH3 mutations are not frequent. The physiological function of IDH is to convert isocitrate into α-KG and generate NADPH. IDH mutations alter the structure of the enzyme active site and the activity of the enzyme. Therefore, upon mutation, IDH1 no longer generates NADPH but consumes NADPH to produce 2-hydroxyglutarate (2-HG). In a normal human body, low levels of 2-HG can be cleared by tissues, but IDH mutations lead to excessive 2-HG production, exceeding the tissue clearance capacity, thereby inducing tumorigenesis.
IDH1 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 20 Sep 2023, there are a total of 24 IDH1 drugs worldwide, from 37 organizations, covering 20 indications, and conducting 90 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.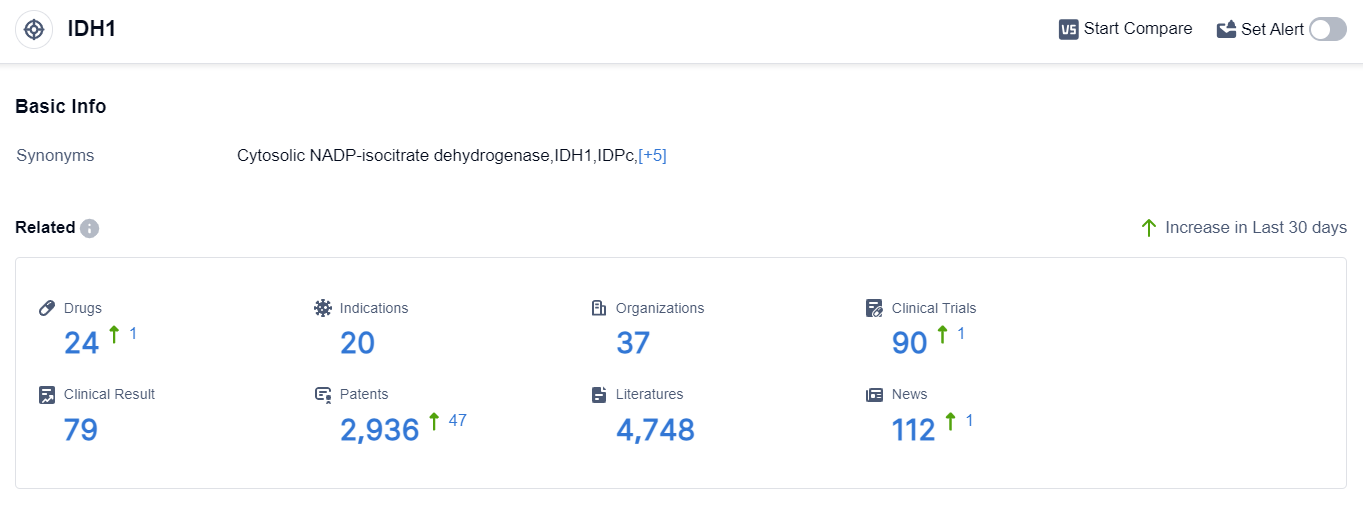
The analysis of target IDH1 reveals a competitive landscape with multiple companies making progress in the development of drugs. Les Laboratoires Servier SAS, CStone Pharmaceuticals Co. Ltd., and Agios Pharmaceuticals, Inc. are among the companies growing fastest under this target.
Drugs targeting IDH1 have been approved for indications such as Acute Myeloid Leukemia (AML), Cholangiocarcinoma, and Glioma. Small molecule drugs are progressing rapidly, indicating intense competition in this area.
The United States, China, and the European Union are the leading regions in terms of R&D progress, with China showing significant progress. The future development of target IDH1 holds promise for the treatment of various cancers and hematologic disorders.
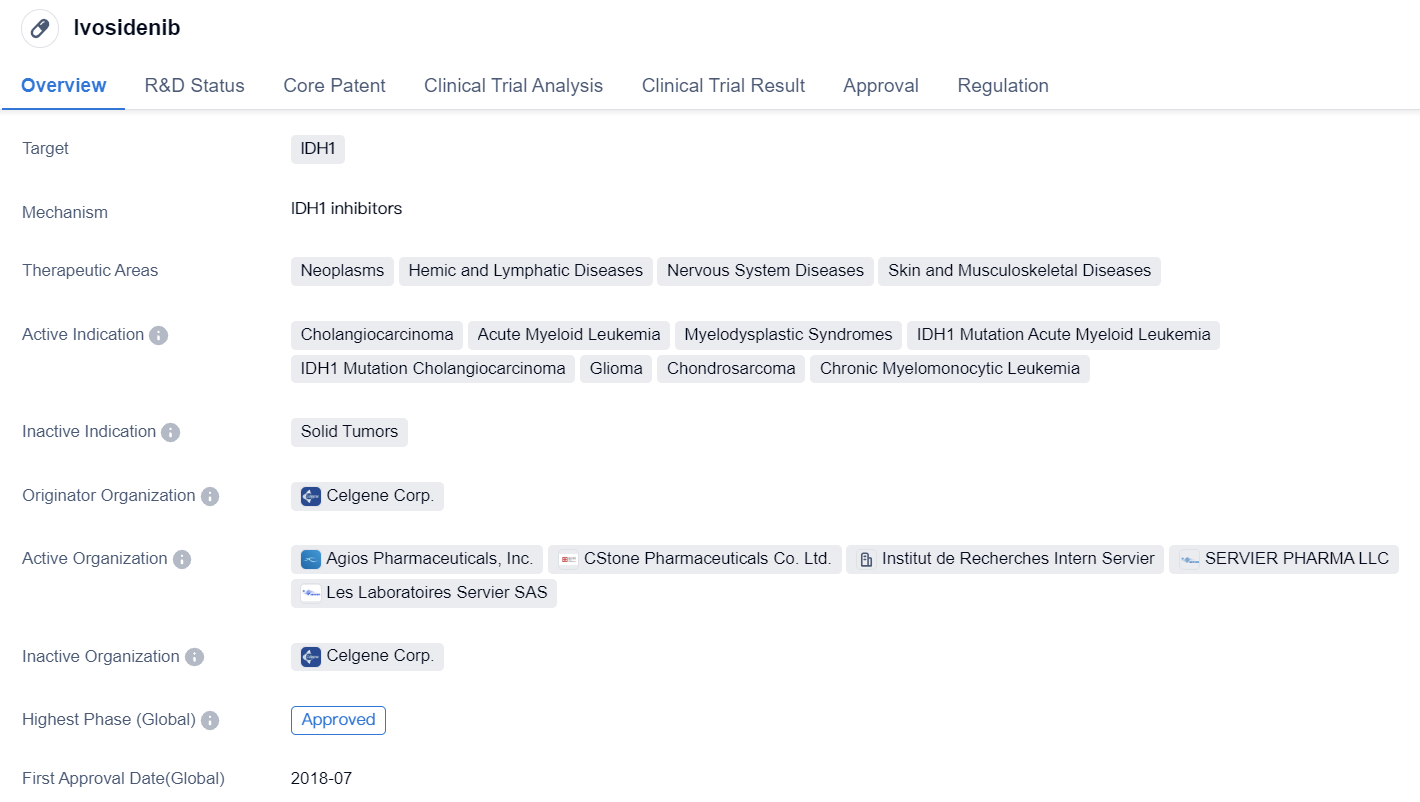
Key Drug: Ivosidenib
Ivosidenib is a small molecule drug that targets IDH1, an enzyme involved in cellular metabolism. It has been developed by Celgene Corp. and has received approval for use in several therapeutic areas, including neoplasms, hemic and lymphatic diseases, nervous system diseases, and skin and musculoskeletal diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug has shown promising results in the treatment of various cancers and related conditions. It has been approved for the treatment of cholangiocarcinoma, a type of bile duct cancer, AML, myelodysplastic syndromes, and glioma, a type of brain tumor. Ivosidenib has also shown potential in the treatment of chondrosarcoma, a type of bone cancer, and chronic myelomonocytic leukemia.
The first approval of Ivosidenib was granted in the United States in July 2018. It has also received approval in China. The drug has undergone rigorous regulatory processes, including priority review, fast track designation, and orphan drug status. These designations highlight the urgent need for new treatment options in clinical settings and the potential benefits Ivosidenib may offer to patients.
The approval of Ivosidenib marks a significant milestone in the field of biomedicine, particularly in the treatment of cancers with IDH1 mutations. By targeting this specific enzyme, Ivosidenib aims to inhibit the abnormal metabolic processes associated with cancer cells, potentially leading to improved patient outcomes.
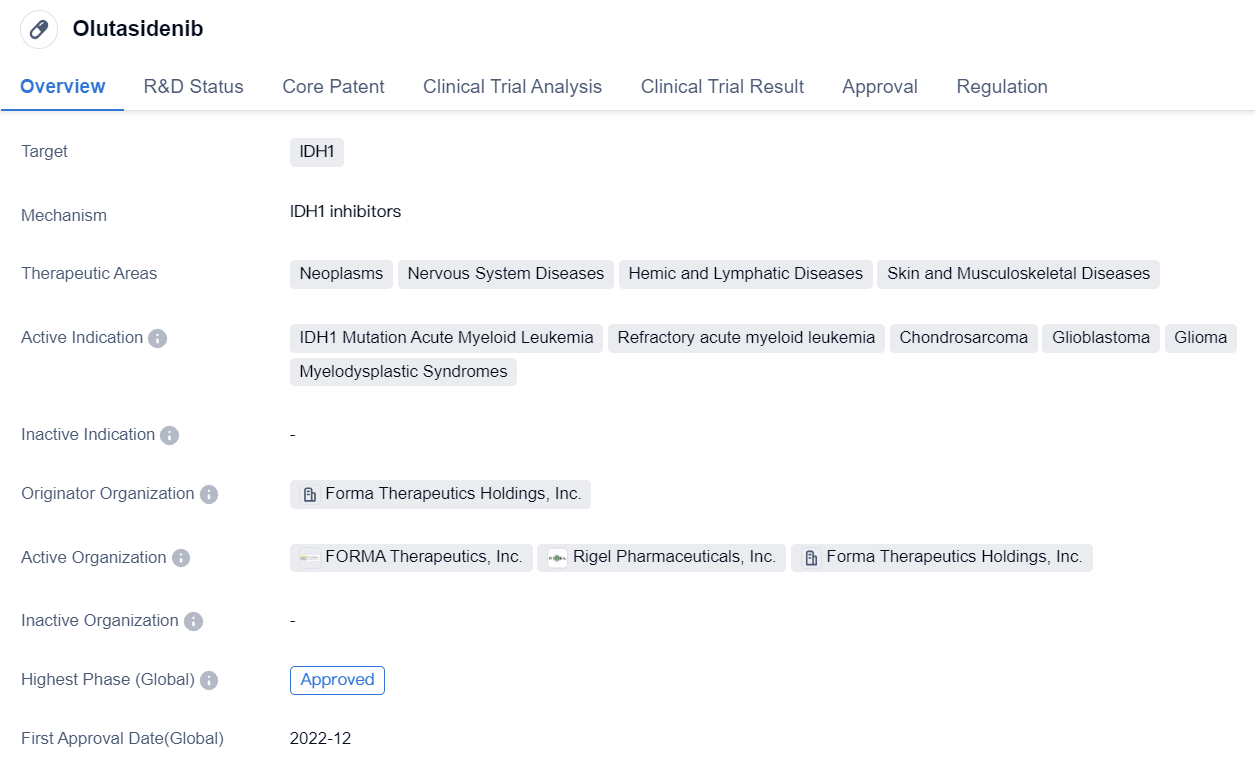
Olutasidenib
Olutasidenib is a small molecule drug that falls under the therapeutic area of biomedicine. It specifically targets IDH1, which is an enzyme involved in cellular metabolism. The drug has shown potential in treating various diseases, including neoplasms, nervous system diseases, hemic and lymphatic diseases, as well as skin and musculoskeletal diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The active indications for olutasidenib include IDH1 mutation AML, refractory AML, chondrosarcoma, glioblastoma, glioma, and myelodysplastic syndromes. These conditions are characterized by abnormal cell growth and can be life-threatening if left untreated.
Forma Therapeutics Holdings, Inc. is the originator organization responsible for the development and production of olutasidenib. As of the latest available information, the drug has reached the highest phase of development, which is approval. This indicates that it has successfully completed clinical trials and has been granted approval for use.
The first approval of olutasidenib was granted in December 2022 in the United States. It is important to note that the drug has been regulated as an orphan drug. Orphan drug status is typically granted to medications that are intended to treat rare diseases or conditions that affect a small number of patients.
In summary, olutasidenib is a small molecule drug developed by Forma Therapeutics Holdings, Inc. It targets IDH1 and has shown promise in treating various diseases, including AML, chondrosarcoma, glioblastoma, glioma, and myelodysplastic syndromes. The drug has received approval in the United States and has been designated as an orphan drug.