Boehringer Ingelheim & Eli Lilly's SGLT2 inhibitor Empagliflozin obtains new indication approval in the European Union
On July 26, 2023, Boehringer Ingelheim and Eli Lilly and Company jointly announced that the European Union has approved Jardiance (empagliflozin) for the treatment of Chronic Kidney Disease (CKD). Empagliflozin is the first Sodium-Glucose Co-Transporter-2 (SGLT2) inhibitor to statistically significantly reduce the risk of hospitalization for any cause compared to a placebo in patients with CKD.
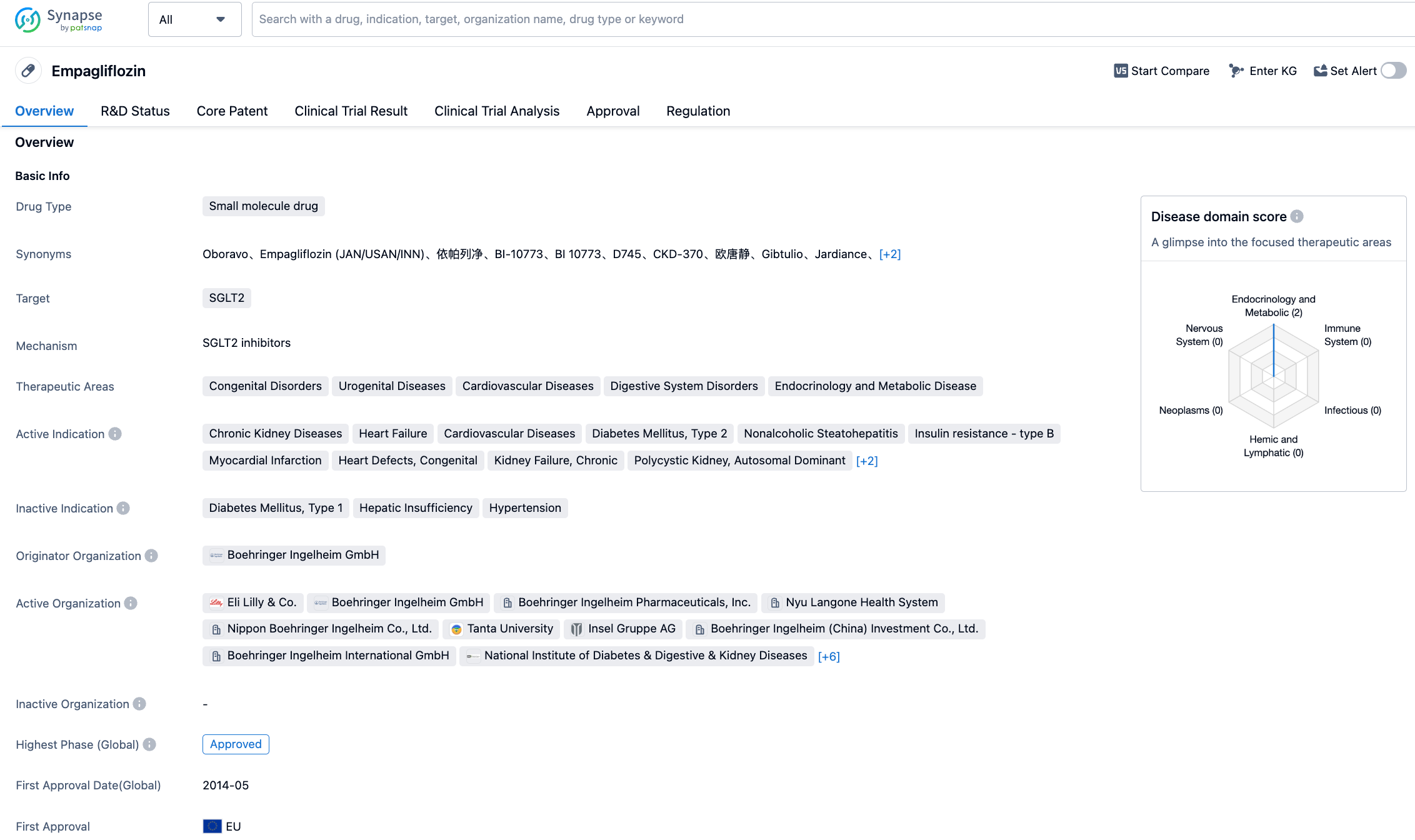
Empagliflozin is a new generation SGLT2 inhibitor co-developed by Boehringer Ingelheim and Eli Lilly. It reduces glucose reabsorption in the kidneys, lowers the renal glucose threshold, and promotes the excretion of glucose in the urine. In addition, empagliflozin can also prevent the reabsorption of salt to increase salt excretion in the body and reduce the fluid load on the body's vascular system. The changes in sugar, salt, and water metabolism induced by empagliflozin help reduce cardiovascular deaths in relevant patients. In the United States, empagliflozin has been approved by the FDA for the treatment of various indications, including type 2 diabetes, to reduce the risk of cardiovascular death in adult patients with type 2 diabetes and cardiovascular disease, and to reduce the risk of cardiovascular death and hospitalization in adult patients with reduced ejection fraction heart failure.
The new indication approval in the EU is based on the EMPA-KIDNEY, a randomised double-blind phase 3 international clinical trial. The trial results showed that compared to placebo, empagliflozin could reduce the risk of progression of kidney disease or cardiovascular death by 28% (HR: 0.72, 95% CI: 0.64-0.82, P<0.000001). Compared to the placebo group, the SGLT2 inhibitor can significantly reduce the risk of hospitalisation due to any cause in patients with chronic kidney disease. Empagliflozin can reduce the risk of hospitalisation by 14% (HR: 0.86, 95% CI: 0.78-0.95, P<0.0025).
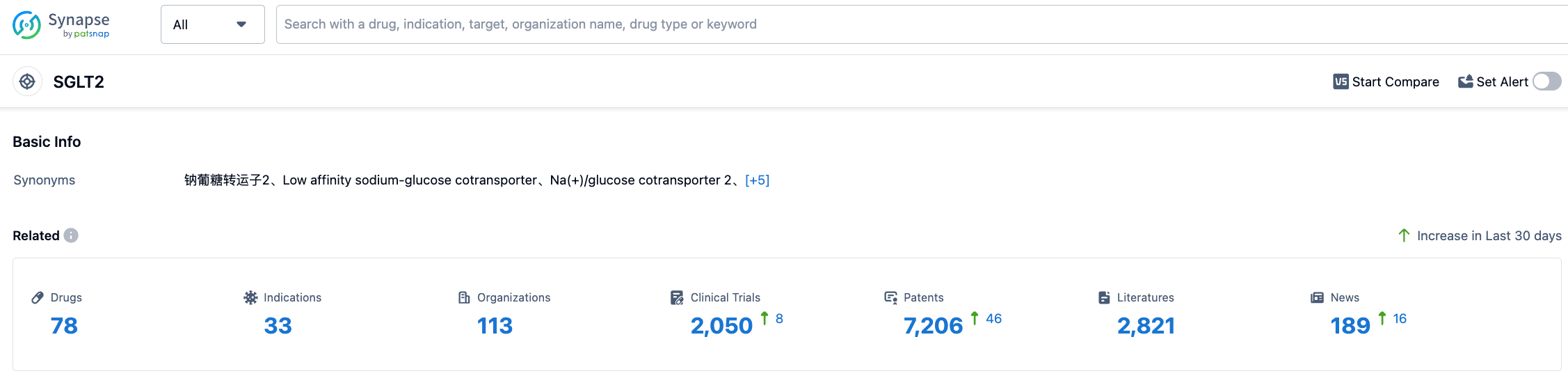
According to the information disclosed by Synapse (click the card below to directly access the SGLT2 target. After registration and login, you can get the detailed information about the investigational drugs, indications, research institutions, clinical trials, etc. under this target for free), as of July 27, 2023, there are a total of 78 investigational drugs for SGLT2 target, covering 33 kinds of indications, with 113 research institutions involved, 2050 related clinical trials, and as many as 7205 patents... We look forward to Empagliflozin playing a bigger role in hypoglycemia, cardiovascular disease, and kidney disease.