The Impact and Development of Sotorasib for KRAS G12C Mutated Cancers
Sotorasib is an oral medication developed to target a specific mutation in the KRAS gene, known as KRAS G12C, which is implicated in various cancers. It is marketed under the brand name Lumakras (or Lumykras in some regions) and is a product of Amgen.
The drug has been granted approval in May 2021 in the United States and is currently in Phase 3 in China. The regulatory status of Sotorasib includes priority review, conditional marketing approval, accelerated approval, fast track, breakthrough therapy designation, and orphan drug status. These designations aim to expedite the development and review process of drugs for serious conditions, meeting an unmet medical need.
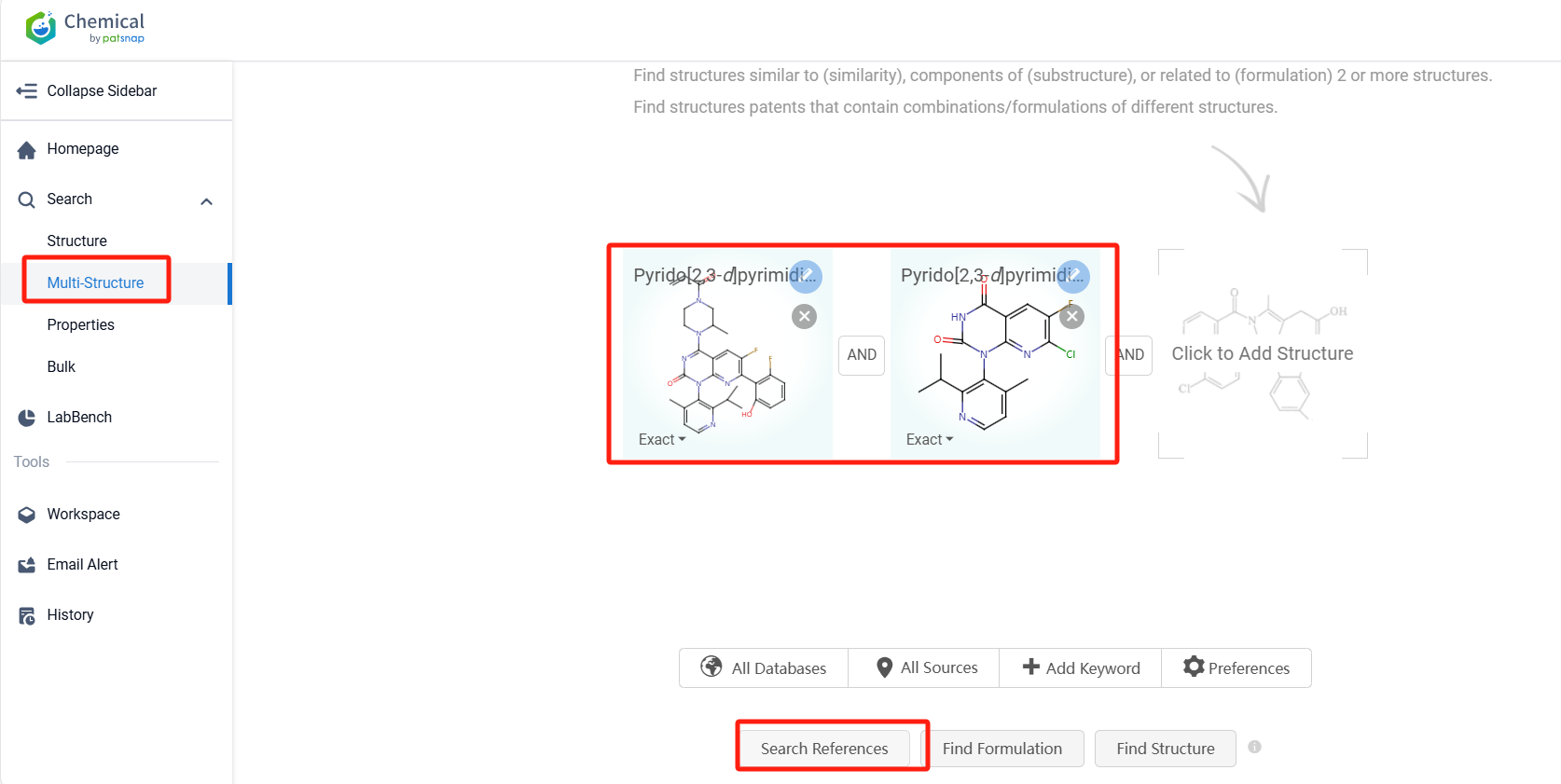
Log in to the Patsnap Chemical. Select the Multi-Structure search, and search for the reactants and products in the specific steps of the synthesis route of Sotorasib. click on search references, and you can query the relevant literature.
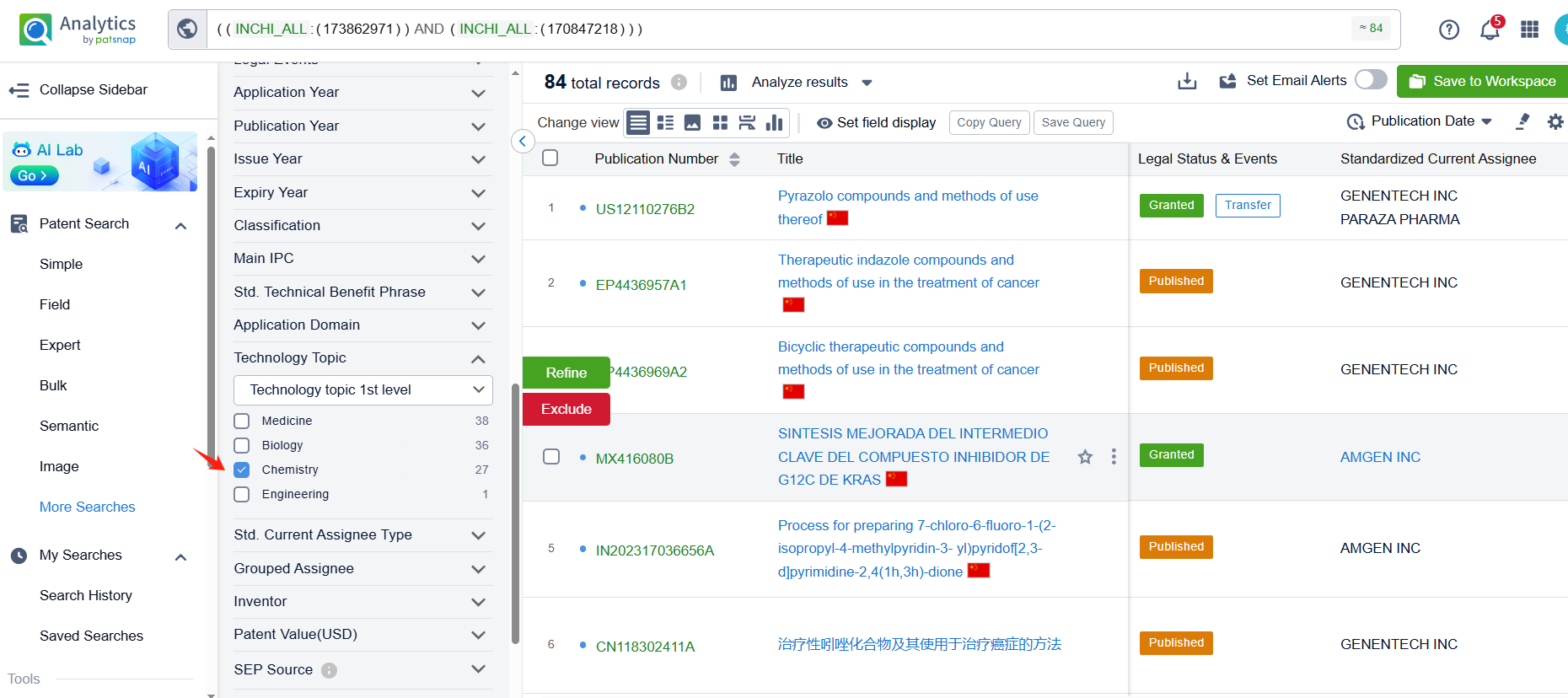
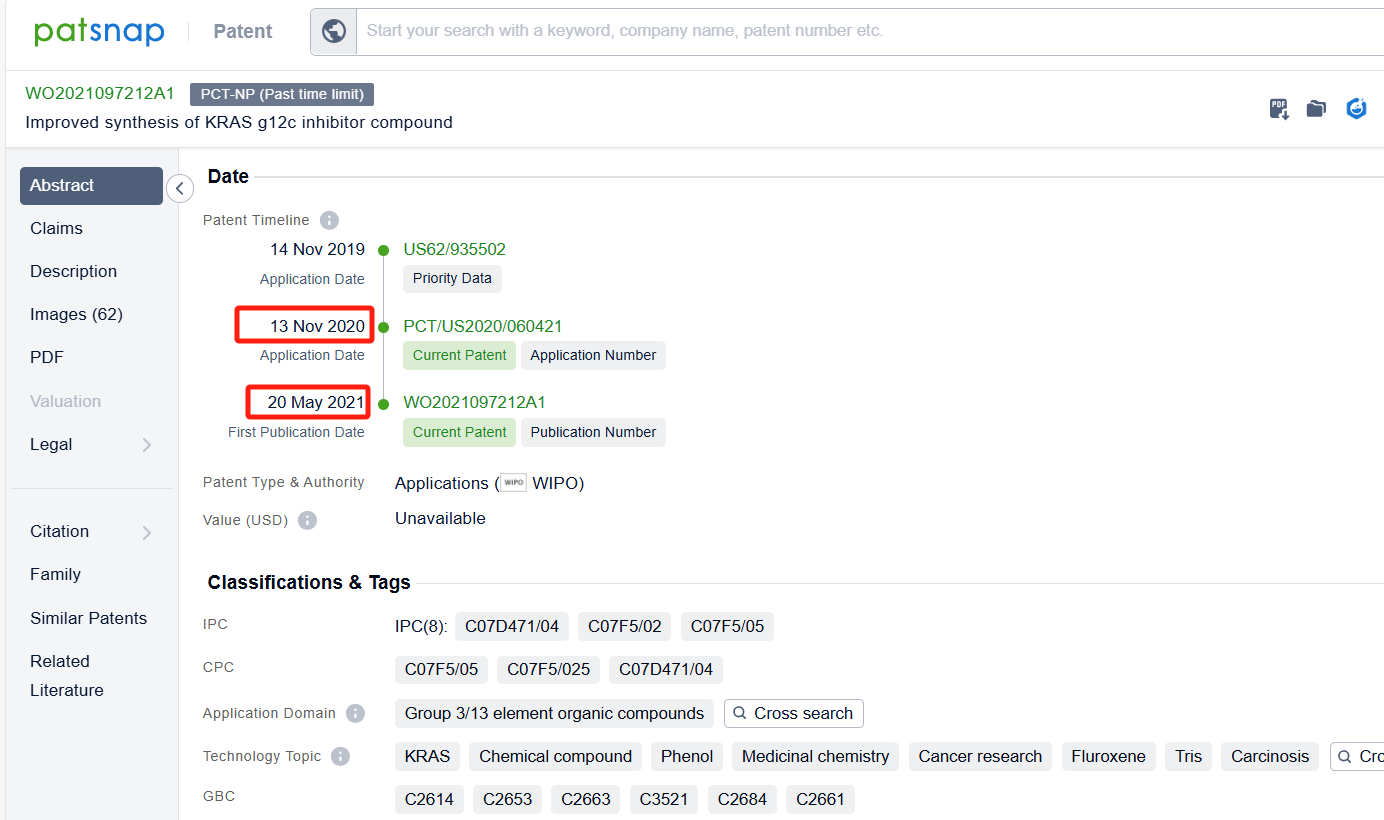
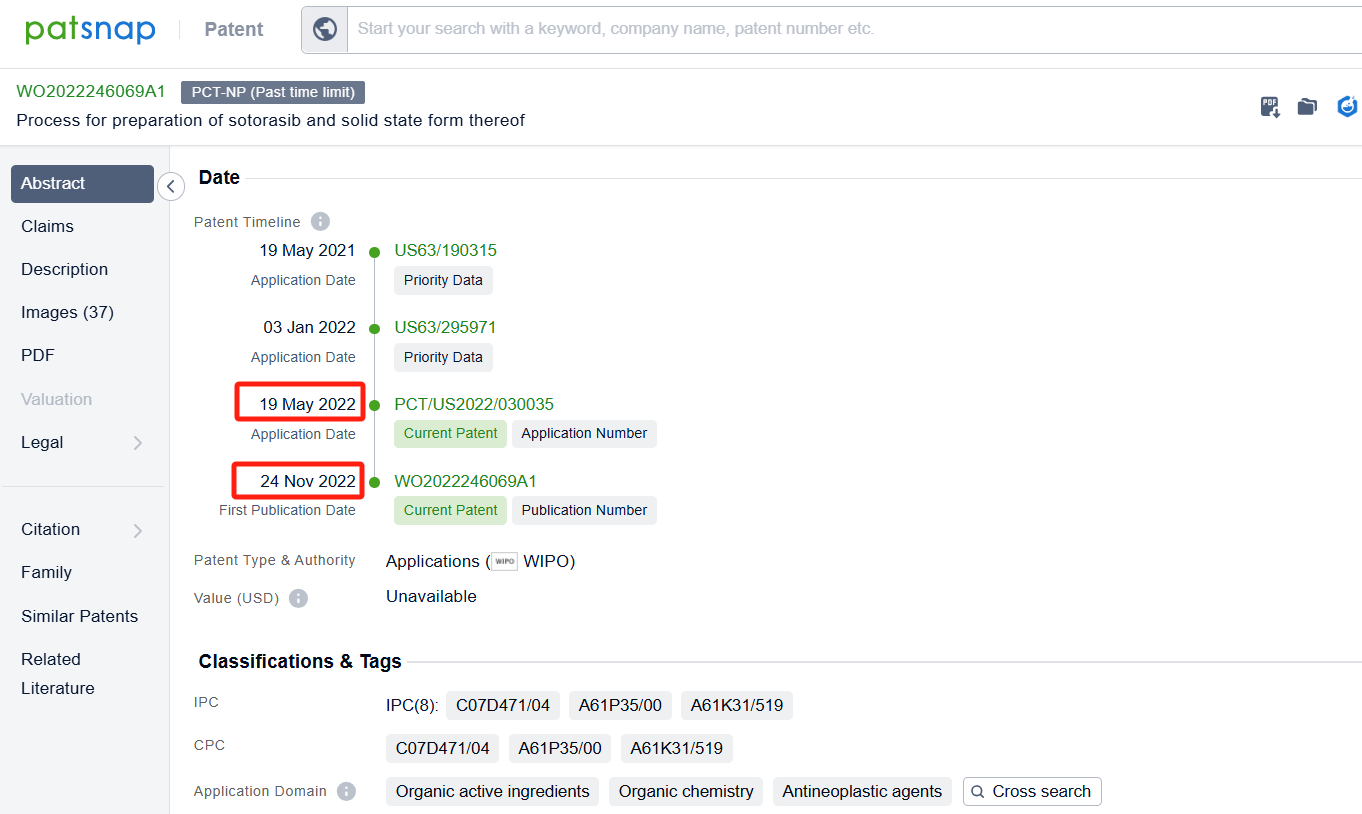
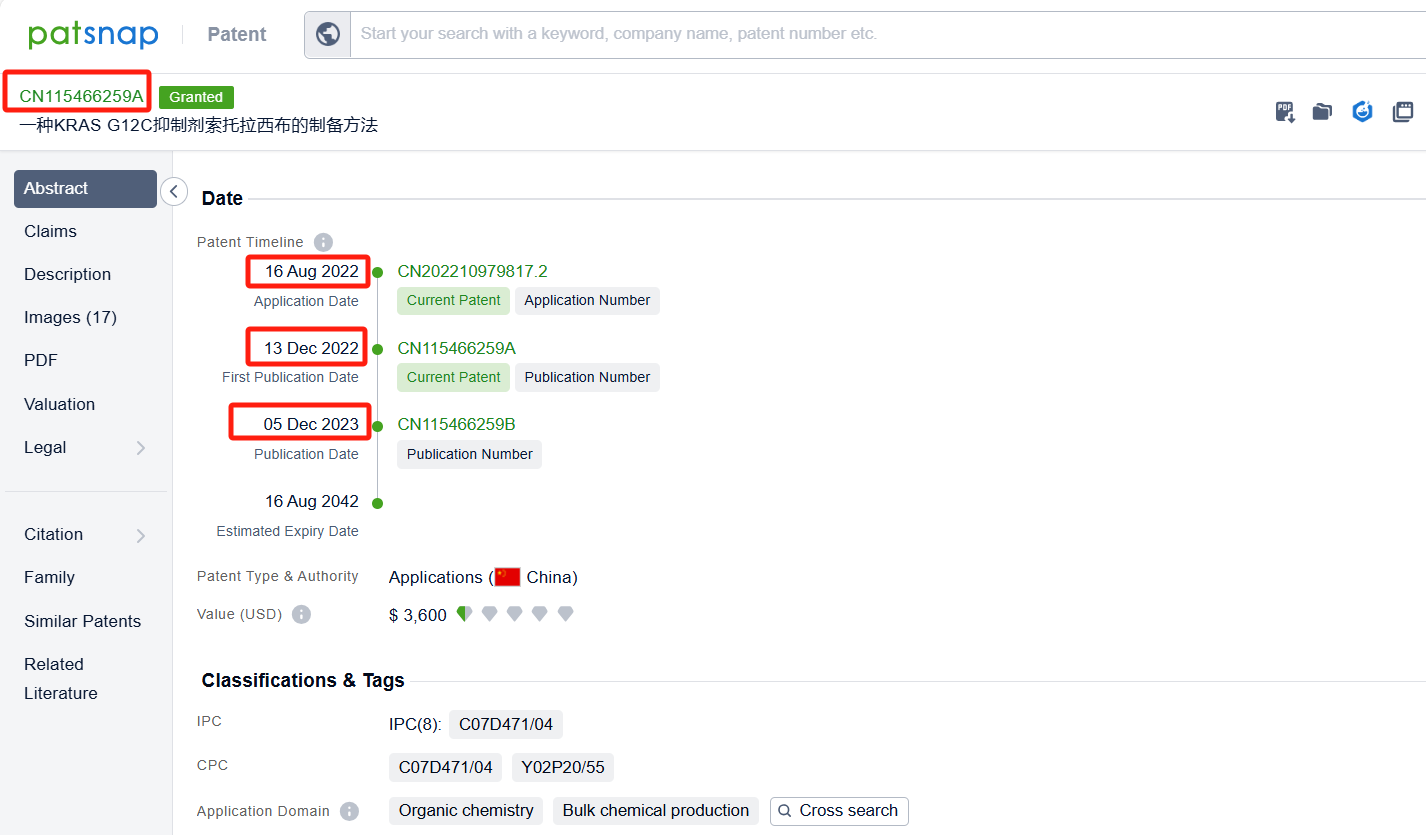
Linked to the Patsnap patent, check the 'Chemistry' category under the technical subject classification and click on the filter, which will allow you to accurately retrieve the design and improvement of the Sotorasib process route by Amgen, Inc. and other companies,Such as Amgen, Inc.'s international patent WO2021097212A1(application date 20201113, publication date 20210520) disclosure relates to an improved, efficient, scalable process to prepare intermediate compounds, useful for the synthesis of Sotorasib for the treatment of KRAS G12C mutated cancers. Teva Pharmaceuticals International GmbH's international patent WO2022246069A1 (application date 20220519, publication date 20221124) relates to an improved process for the preparation and atropoisomeric separation of key intermediates in the process of making of Sotorasib and salts thereof. Additionally, Shandong University's patent CN115466259A (application date 20220816, publication date 20221213) disclosure a preparation method of KRAS G12C inhibitor Sotoracib, The present invention studies the synthesis process of the intermediate 2,6-dichloro-5-fluoronicotinic acid amide, optimizes the reaction steps and post-treatment methods, and improves the yield. The patent was granted on December 5, 2023.
The approval of Sotorasib marks a significant advancement in the treatment of various cancers associated with the KRAS G12C mutation, providing a new treatment option for patients with limited therapeutic alternatives. The drug's approval in the United States reinforces its safety and efficacy profile, with ongoing studies in China potentially expanding its global reach.
The therapeutic potential of Sotorasib in addressing the challenging landscape of KRAS G12C mutations in different cancer types underscores its significance in precision medicine and personalized treatments. As Sotorasib continues to progress through clinical trials and regulatory pathways, it represents a promising addition to the armamentarium of therapies for these difficult-to-treat malignancies.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.