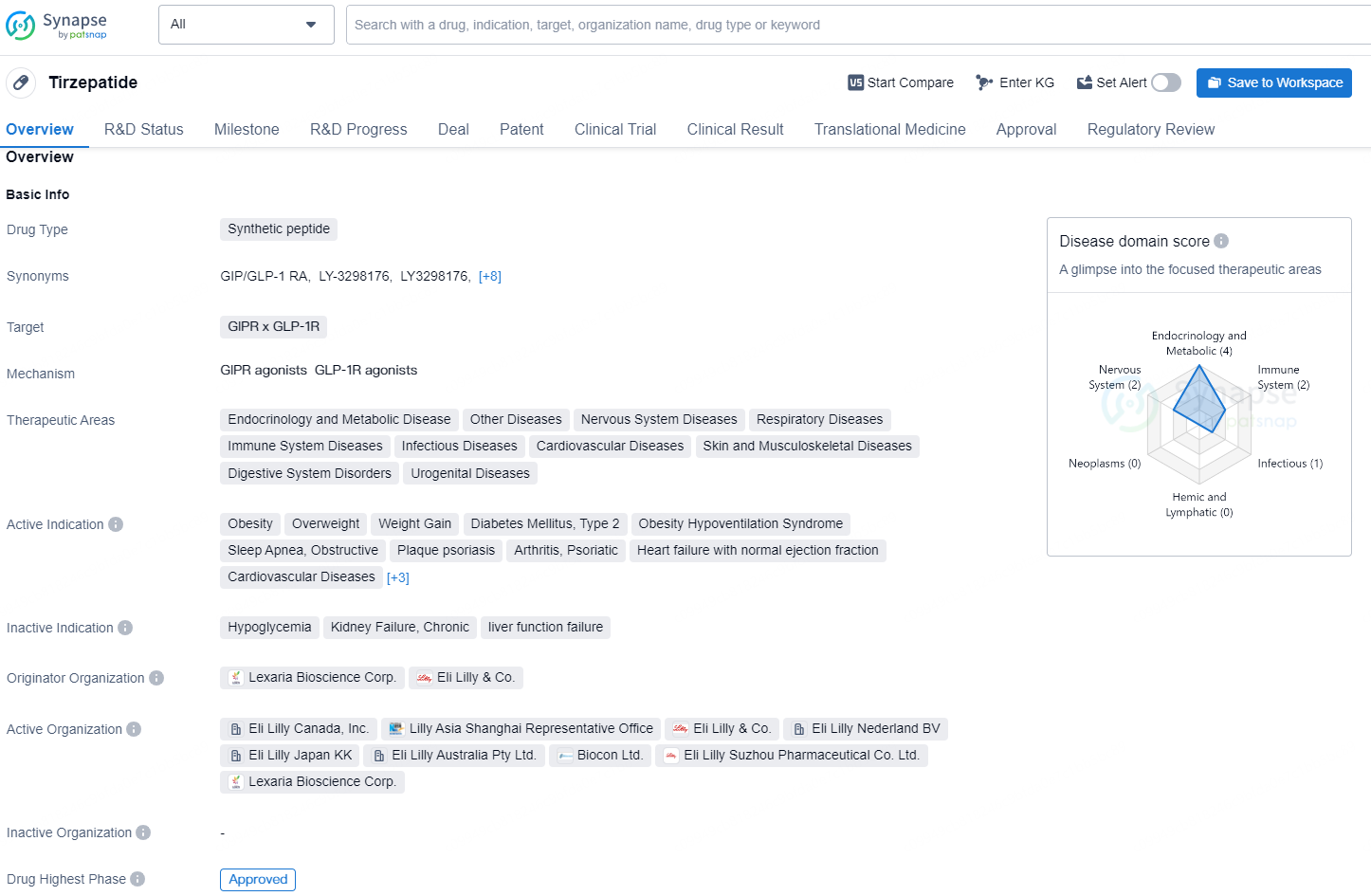
Tirzepatide Achieves Long-term Weight Loss and Diabetes Prevention in Overweight Adults
Eli Lilly and Company (NYSE: LLY) has released comprehensive findings from the Phase 3 SURMOUNT-1 study, which lasted three years (176-week treatment duration), marking it as the longest study conducted on tirzepatide to date. The administration of weekly tirzepatide injections (comprising pooled doses of 5 mg, 10 mg, and 15 mg) significantly lowered the likelihood of developing type 2 diabetes in adults suffering from pre-diabetes and obesity or being overweight, in contrast to placebo, over the span of 176 weeks. The 15 mg dose of tirzepatide was associated with an average sustained weight reduction of 22.9% throughout the entire three-year period among the efficacy estimandi. These results were published in The New England Journal of Medicine (NEJM) and were recently showcased at ObesityWeek 2024.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
According to Ania Jastreboff, M.D., Ph.D., director of the Yale Obesity Research Center, “Patients receiving tirzepatide experienced an average weight loss of up to 23% and sustained this reduction for over three years, significantly lowering their risk of developing type 2 diabetes. Remarkably, approximately 99% of those treated with tirzepatide remained diabetes-free after 176 weeks.” She noted, “These findings are noteworthy due to the sustained loss of weight and the corresponding decrease in diabetes risk.”
Tirzepatide is recognized as the first and only authorized dual agonist for the GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) receptors. GIP and GLP-1 are hormones produced by the intestines in response to food intake and play a key role in the incretin effect.
Jeff Emmick, M.D., Ph.D., senior vice president of product development at Lilly, stated, “In the three-year SURMOUNT-1 trial, participants lost an average of 22.9% of their weight, with a hazard ratio of 0.06 indicating progression to type 2 diabetes. This represents a 94% reduction in risk, requiring only nine individuals to treat in order to prevent one case of diabetes.” He emphasized, “These findings highlight the importance of long-term treatment with effective medications like tirzepatide for achieving and sustaining weight loss.”
Additional outcomes from the study linked tirzepatide treatment to enhancements in glycemic control, improvements in cardiometabolic risk factors (such as fasting insulin levels, blood pressure, and lipid profiles), as well as a better health-related quality of life sustained throughout the 176 weeks. A post hoc mediation analysis indicated that roughly half of the observed delay in the onset of type 2 diabetes associated with tirzepatide could be attributed to weight loss induced by the medication, while the remaining benefits may relate to other effects of tirzepatide.
The safety and tolerability of tirzepatide over 193 weeks (which included 176 weeks of treatment followed by 17 weeks off) were consistent with previous findings after 72 weeks from the SURMOUNT-1 trial and other studies on tirzepatide for weight management and long-term maintenance. Apart from COVID-19, the most commonly reported side effects were gastrointestinal in nature, typically mild to moderate in intensity. The most prevalent gastrointestinal issues among patients taking tirzepatide included nausea, diarrhea, and constipation.
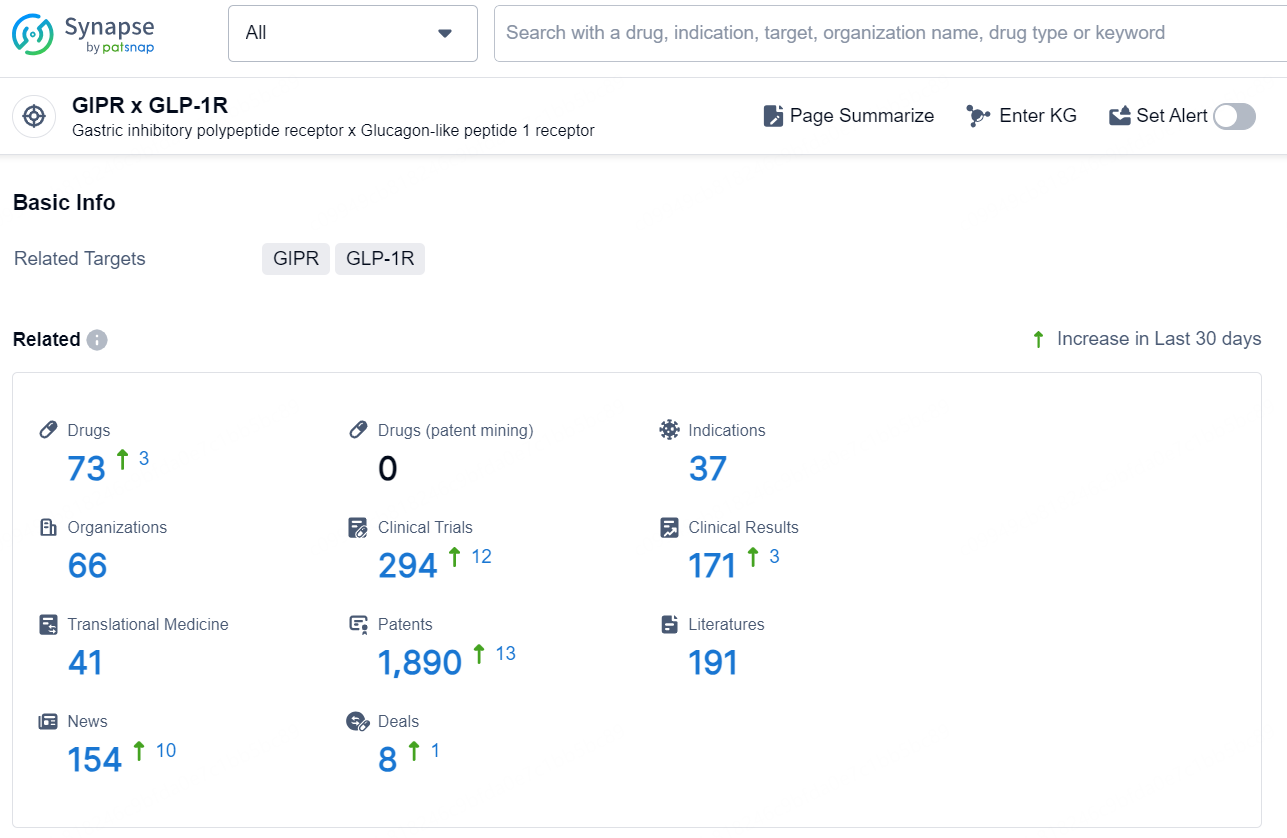
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of November 18, 2024, there are 73 investigational drugs for the GIPR and GLP-1R target, including 37 indication, 66 R&D institutions involved, with related clinical trials reaching 294, and as many as 1890 patents.
Tirzepatide is a novel medication designed to manage type 2 diabetes and potentially treat obesity. It belongs to a new class of drugs known as dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonists. These hormones are part of the incretin system, which helps regulate blood sugar levels and appetite.