AskBio begins Phase 1 trial with first patient for their MSA-P gene therapy, AB-1005
Diary of Bayer AG and independent gene therapy firm, Asklepios BioPharmaceutical, Inc., declared that the Ohio State University Wexner Medical Center has successfully randomized its first patient in the Phase 1 REGENERATE MSA-101 clinical trial. The test concerns AB-1005, a gene therapy that is under development to potentially treat multiple system atrophy-parkinsonian type.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The development of AB-1005 gene therapy, a therapy involving an adeno-associated viral vector that enables the production of glial cell line-derived neurotrophic factor in the putamen, takes a meaningful leap towards potentially benefiting patients. Currently, AB-1005 is also being tested as a possible treatment for mild to moderate Parkinson's disease, with the completion of Phase 1b study participant recruitment.
Several individuals related to the MSA community view the enrollment of the initial participant in the REGENERATE MSA-101, a Phase 1 trial, as a noteworthy occasion, as expressed by Philip M. Fortier, MA, President and Executive Director of the Defeat MSA Alliance.
In his words, "MSA currently has no known cure, nor are there treatments that can halt or slow its progression. This situation is particularly challenging for patients as they often witness a fast deterioration in their condition. The current progress is henceforth seen as a significant step towards potentially altering the prognosis for MSA patients."
MSA-P, often challenging to differentiate from Parkinson's disease, commonly exhibits symptoms like slowed movement, imbalance, unsteady coordination, dizziness, and further difficulties in motor activities. This is primarily caused by a gradual loss of nerve cells in the brain and spinal cord. With an estimated global prevalence of 100,000-500,000, MSA is a rare disease that reportedly appears without an apparent cause. Symptoms generally begin to manifest in patients in their 50s and usually show swift progression within 5-10 years.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
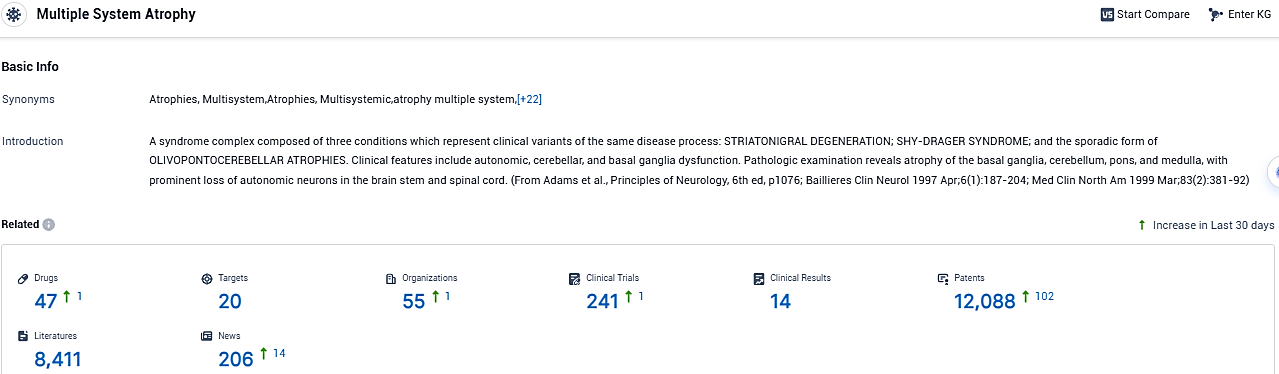
According to the data provided by the Synapse Database, As of November 26, 2023, there are 47 investigational drugs for the multiple system atrophy, including 20 targets, 55 R&D institutions involved, with related clinical trials reaching 241, and as many as 12088 patents.
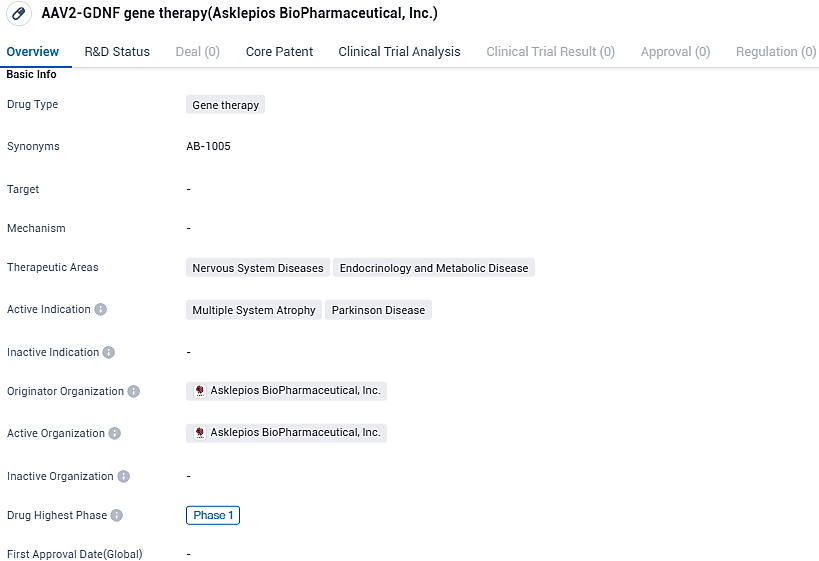
AB-1005 is a gene therapy drug targeting nervous system diseases and endocrinology and metabolic diseases. Its active indications include multiple system atrophy and Parkinson's disease. As the drug is currently in Phase 1 of clinical development, further research and evaluation are needed to determine its safety and efficacy in treating these condition.