The reason of Synthesis of oligonucleotides is an important part of several recent experiments in genetics
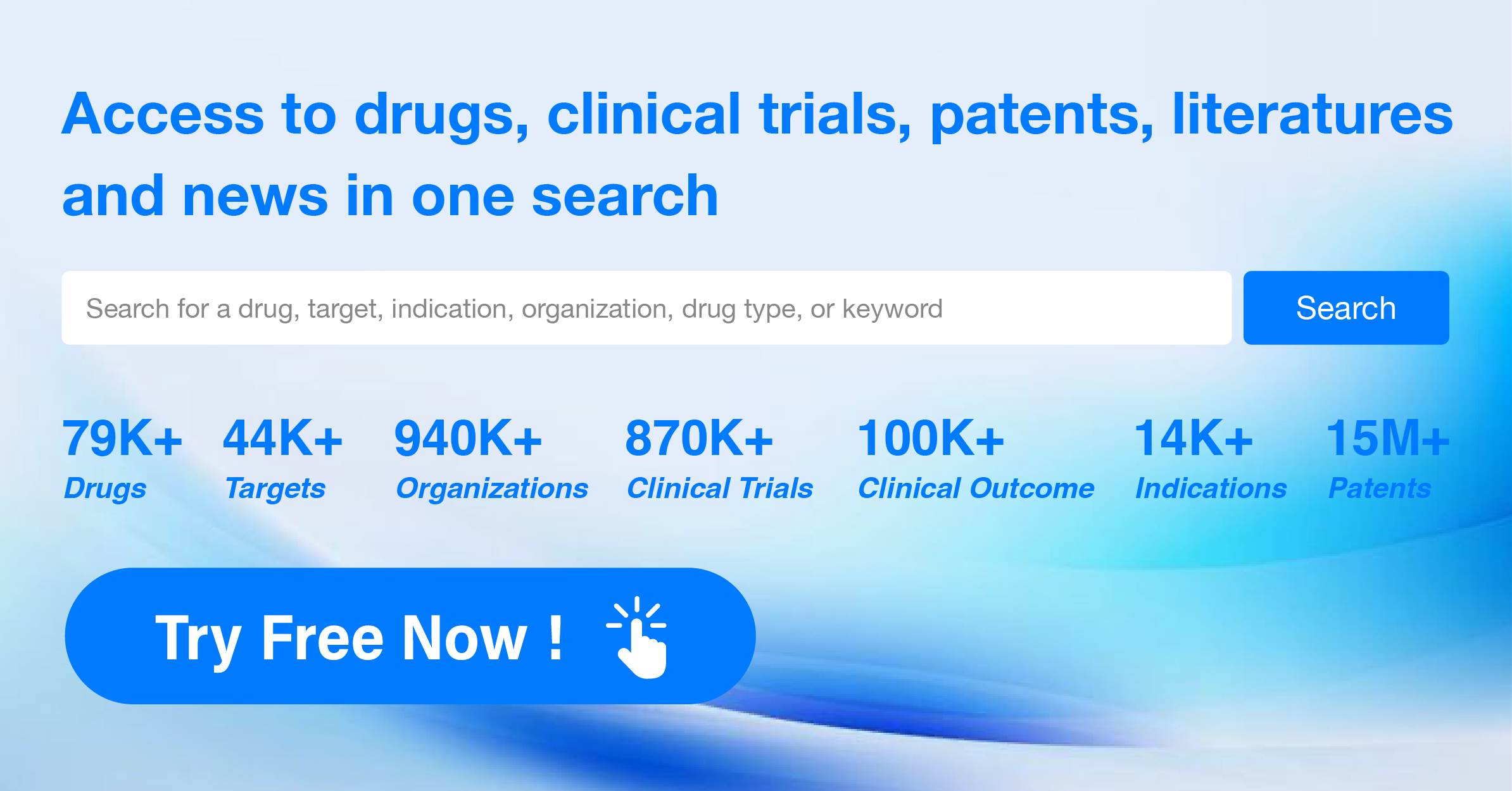
Decreasing sequencing costs have contributed to increasing demand in science, genetic testing, drug development and forensic applications of custom-made nucleotides for desirable series. In turn, this promoted demand in the various life-science industries for oligonucleotides synthesis. Custom synthesis requests from various classes of oligos including decoies, aptamers and immuno-stimulants, antisense, siRNA and miRNA are projected to increase investment in genetic research. Most big and small suppliers of oligonucleotides grow their market by partnering with drug manufacturers. CordenPharma International, for instance, formed a strategic partnership with GE Healthcare Dharmacon Inc. in June 2017 to establish an end-to-end drug discovery approach for oligonucleotides.
Late clinical trials addressing various conditions, for instance diabetes, heart problems, muscular dystrophies and eye defects are being performed with a substantial number of oligos. This anticipated regulatory approval is expected to promote the market for multiple oligonucleotide based therapies. Direct sales and sales through distributors in specific countries are part of marketings implemented in this market. The market is expected to benefit from constant growth in the genomics and genetic modification markets as progress in genetic instruments simplifies oligonucleotide synthesis.