Everest Pharma Receives Approval for Clinical Trial Application of Zetomipzomib in China
Everest Medicines, a company specialized in the biopharmaceutical field dedicated to advancing, producing, and marketing novel therapeutic drugs and immunizations, has disclosed that the regulatory authority in China, the China National Medical Products Administration, has officially acknowledged the submission of their Investigational New Drug (IND) application pertaining to the drug candidate zetomipzomib within the Chinese jurisdiction.
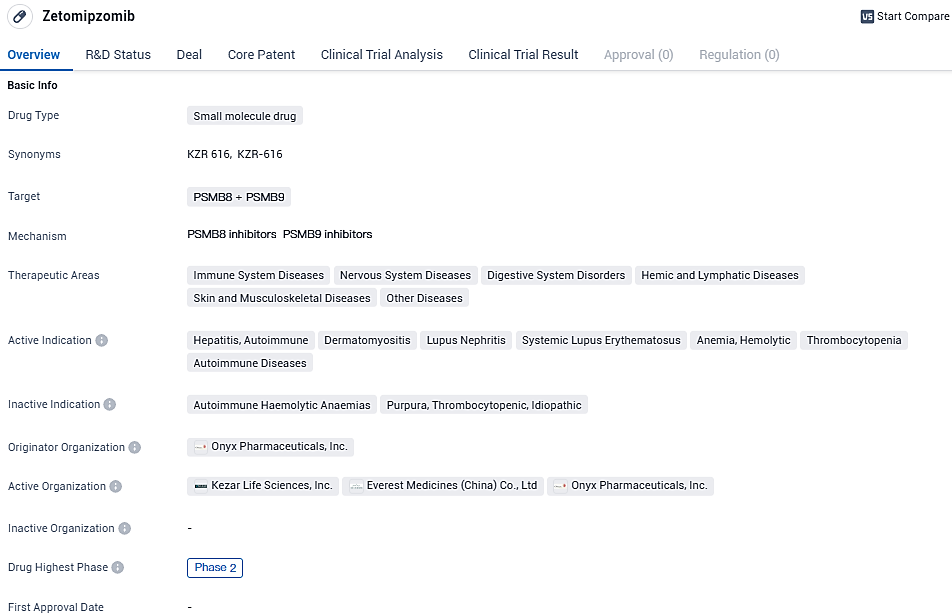
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Zetomipzomib is a groundbreaking, premier molecule designed as a targeted inhibitor of the immunoproteasome, and it is under investigation for its potential to treat various diseases that are driven by the immune system, such as lupus nephritis.
Everest is poised to work alongside its collaborator, Kezar Life Sciences, to partake in the international, randomized, placebo-managed Phase 2b PALIZADE study. This study is intended to explore the therapeutic benefits and safety profile of zetomipzomib at two different dosing regimens in individuals affected by severe lupus nephritis (LN). LN stands as a prevalent immune-related glomerular disorder that may progressively cause renal impairment. It is estimated that there are between 400,000 and 600,000 individuals with LN within Chinese territories.
The approval of zetomipzomib's Investigational New Drug (IND) application is a significant milestone that paves the way for clinical trials within China. "We are eager to contribute to the PALIZADE study by recruiting patients with LN from areas where the condition is notably widespread," expressed Rogers Yongqing Luo, Chief Executive Officer at Everest Medicines.
Luo further remarked, "Targeting illnesses affecting the kidneys and immune system is central to Everest's objectives. With zetomipzomib being a potential key product in our portfolio at an advanced developmental phase, we expect to strengthen our foothold in these specialized medical fields across Asia."
Preliminary findings from a preliminary Phase 2 investigation of zetomipzomib reported promising outcomes. By week 25, the general renal response rate stood at 64.7%, escalating to 88.2% by week 37. The rate of complete renal response was observed at 35.3% during the 25-week mark and rose slightly to 41.2% at week 37. Additionally, there was a notable mean decrease of 57.0% in the urine protein creatinine ratio compared to the initial values at week 25, which further improved to an 83.0% reduction by week 37. Concurrently, the estimated glomerular filtration rate was maintained throughout the course of treatment.
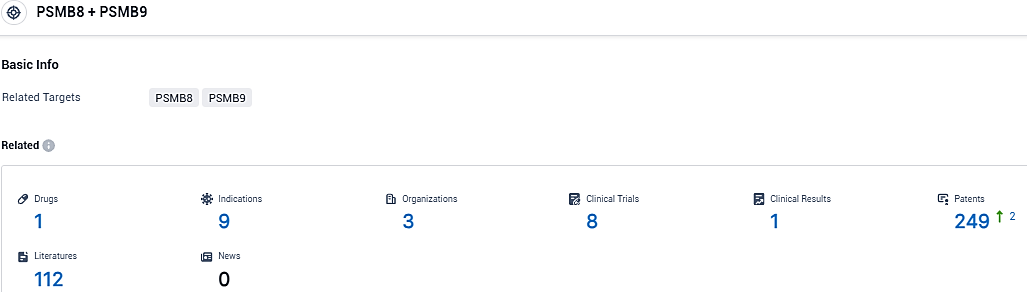
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 12, 2023, there are 1 investigational drugs for the PSMB8 and PSMB9 target, including 9 indications, 3 R&D institutions involved, with related clinical trials reaching 8, and as many as 249 patents.
Zetomipzomib targets PSMB8 and PSMB9 proteins and has shown potential therapeutic applications in various immune-related and autoimmune diseases. The drug has reached Phase 2 of clinical development globally and has submitted an IND application in China. Further clinical trials will be needed to evaluate its safety and efficacy before it can be approved for commercial use.