4DMT reports its first patient for the 4D-150 Phase 2 SPECTRA Clinical Examination in DME
The clinical-phase biotherapeutics company, 4D Molecular Therapeutics, that utilizes directed evolution for precision genetic treatments, has started the Dose Confirmation phase of their Phase 2 SPECTRA clinical trial with the enrollment of their first patient. The trial is assessing intravitreal 4D-150 for treating patients suffering from diabetic macular edema. Additionally, a Population Extension group has been incorporated into the Phase 2 stage of 4D-150 PRISM Clinical Trial for wet age-related macular degeneration.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"We are excited about progressing 4D-150 in our Phase 2 SPECTRA trial, which involves DME patients, while simultaneously broadening the patient group for intravitreal 4D-150 targeting wet AMD," declared Dr. Robert Kim, the 4DMT medical director. "DME is a significant contributing factor to vision impairments in diabetes patients, leading potentially to blindness. We are hopeful that through expanding our pool of patients being treated with 4D-150 for wet AMD, we can further understand 4D-150 prior to initiating our anticipated Phase 3 clinical studies.”
The swift progression of 4D-150 in DME and wet AMD emphasize our dedication to high-impact genetic therapies in ophthalmology for patients in need, and display the intrigue of doctors and patients towards the transformational potential of a one-time intravitreal 4D-150 dose,” stated Dr. David Kirn, Co-founder and CEO of 4DMT.
DME presents a considerable opportunity for 4DMT, and the distinct status of 4D-150 in wet AMD is predicted to bolster interest and substantial enrollment in the SPECTRA trial. In wet AMD, the encouraging clinical character of 4D-150 in patients with a high need for anti-VEGF has facilitated the expanding of the PRISM trial to incorporate a more diverse patient group in the Population Extension cohort.
4D-150 comprises our tailored and advanced intravitreal vector, R100, as well as a transgene cassette that expresses aflibercept alongside a VEGF-C inhibitory RNAi. This two-transgene payload suppresses 4 angiogenic factors that stimulate wet AMD and DME: VEGF A, B, C, and PGF. R100 was invented at 4DMT through our proprietary Therapeutic Vector Evolution platform; we created this platform utilizing principles of directed evolution, a Nobel Prize-winning technology. The design of 4D-150 allows for a single, low-dose intravitreal administration.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
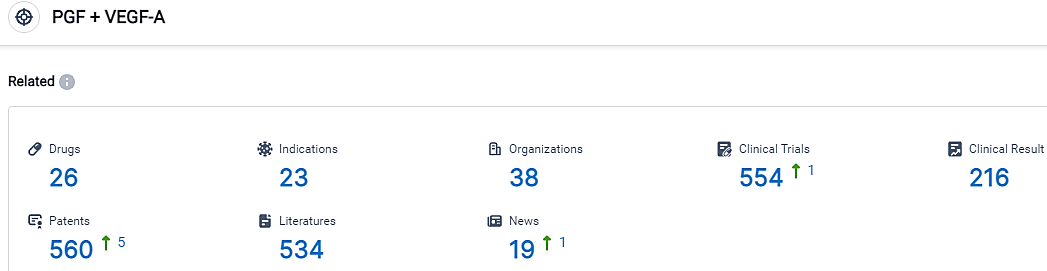
According to the data provided by the Synapse Database, As of September 12, 2023, there are 26 investigational drugs for the PGF and VEGF-A target, including 23 applicable indications,38 R&D institutions involved, with related clinical trials reaching 554,and as many as 560 patents.
The current competitive landscape of the indication Diabetic macular oedema is dominated by companies such as Novartis AG, Roche Holding AG, Regeneron Pharmaceuticals, Inc., and Bayer AG. The most common target for DME drugs is VEGF-A, followed by other targets such as GR, PGF + VEGF-A, Ang2 + VEGF-A, VEGFR, and others. Overall, the future development of DME is promising, with ongoing research and development efforts focused on innovative drugs and competition in the market.