VBI Vaccines starts administering their first doses to patients in the Phase 2b trial of VBI-1901
Immunology-focused biopharma corporation VBI Vaccines Inc. has declared that initial patients have received dosages in the regulated, randomized Phase 2b trial involving VBI's cancer immunotherapy vaccine candidate, VBI-1901, for individuals who are experiencing first recurrence glioblastoma. This research intends to evaluate the safety, patient tolerance, tumor reaction rates, and the aftermath on survival subsequent to VBI-1901 treatment on its own, at 10 leading neuro-oncology centers across the United States.
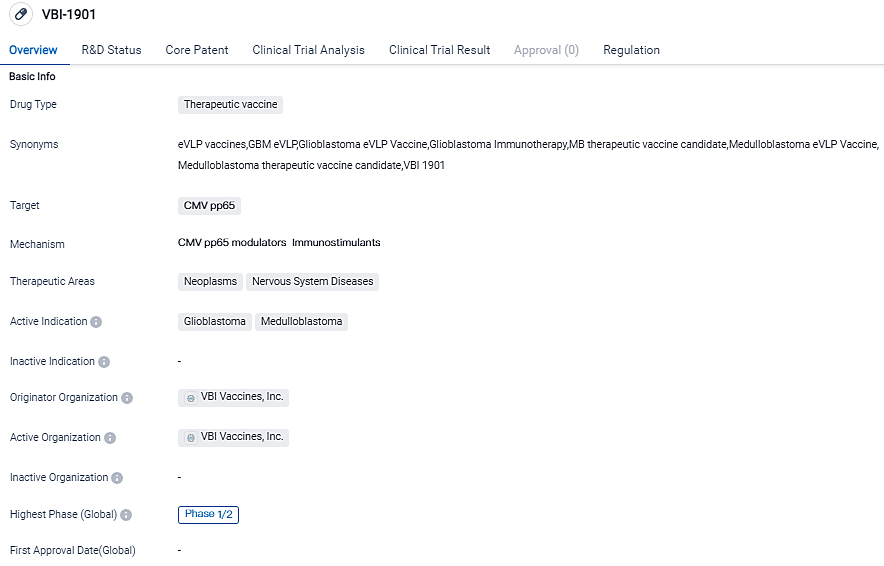
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Dr. Andrew B. Lassman, who holds the position of Neuro-Oncology Chief at Columbia University Vagelos College of Physicians and Surgeons and acts as the primary investigator of this research, shared his thoughts, "Considering the early and preclinical findings of this trial, I am eager to showcase the potential advantages of VBI-1901 compared to existing treatments in the upcoming clinical stage. The aim is to make a significant difference in tackling an infamously dangerous brain tumor with a high mortality rate."
Adding his views, Dr. Francisco Diaz-Mitoma, Ph.D., the Chief Medical Officer at VBI, commented, "The average survival rate in recurrent GBM cases stands at merely eight months, necessitating any improvements in patient care. While we strive to bring renewed optimism for patients, their families and caretakers dealing with this historically challenging brain tumor, we are thrilled to start the next phase of VBI-1901 development."
The comprehensive biomarker panels proposed in the Phase 1/2a VBI-1901 trial for rGBM revealed typical baseline CD4+/CD8+ T-cell ratios, which serve as an immunological health indicator. Correspondingly, an increase in cytomegalovirus gB unique antibody responses was associated with tumor and clinical outcomes. Such insights have been factored into patient admission criteria for the Phase 2b trials to pinpoint patient groups possibly more responsive to VBI-1901.
VBI-1901 is a novel cancer vaccine immunotherapy candidate, conceived using VBI's encapsulated virus-like particle technology. This targets two highly immunogenic cytomegalovirus (CMV) antigens, gB and pp65. The FDA has granted VBI-1901 the Fast Track Designation and Orphan Drug Designation for the treatment of recurrent glioblastoma. These designations aim to confer certain benefits to drug inventors, like more frequent engagement with the FDA and Accelerated Approval and Priority Review, provided specific conditions are fulfilled, among other advantages.
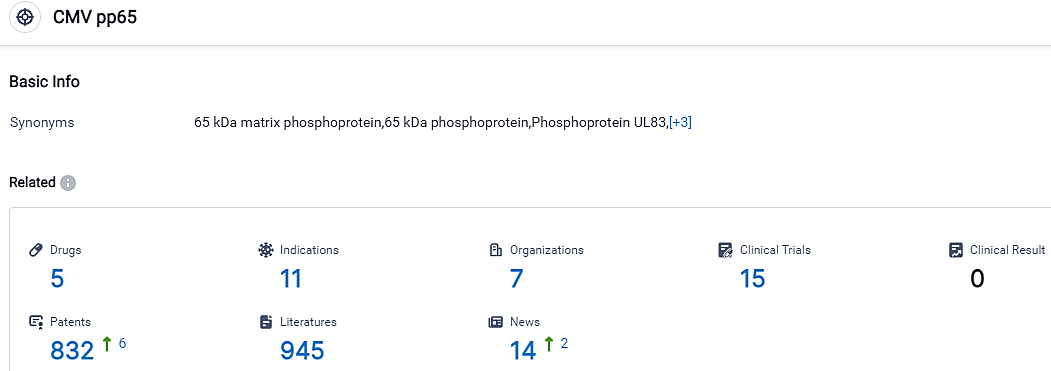
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 12, 2023, there are 5 investigational drugs for the CMV pp65 target, including 11 applicable indications,7 R&D institutions involved, with related clinical trials reaching 15,and as many as 832 patents.
Scientific literature suggests CMV infection is prevalent in multiple solid tumors, including glioblastoma. GBM is among the most common and aggressive malignant primary brain tumors in humans. In the U.S. alone, 14,000 new cases are diagnosed each year. The current standard of care for treating GBM is surgical resection, followed by radiation and chemotherapy. Even with aggressive treatment, GBM progresses rapidly and has a high mortality.