TiumBio Files for Phase 1b Trial of Long-Lasting Factor VII TU7710 in Hemophilia A/B Patients
TiumBio Co., Ltd., an emerging leader in the biotech industry dedicated to the pursuit of groundbreaking treatments for individuals battling uncommon and relentless illnesses, recently revealed its submission for a Clinical Trial Application to both the Italian Medicines Agency and the Spanish Agency of Medicines and Medical Products. The aim is to embark on a Phase 1b clinical trial of TU7710, an innovative form of recombinant activated factor VII (rFVIIa) specifically designed for use in hemophilia sufferers who have developed resistance through inhibitors.
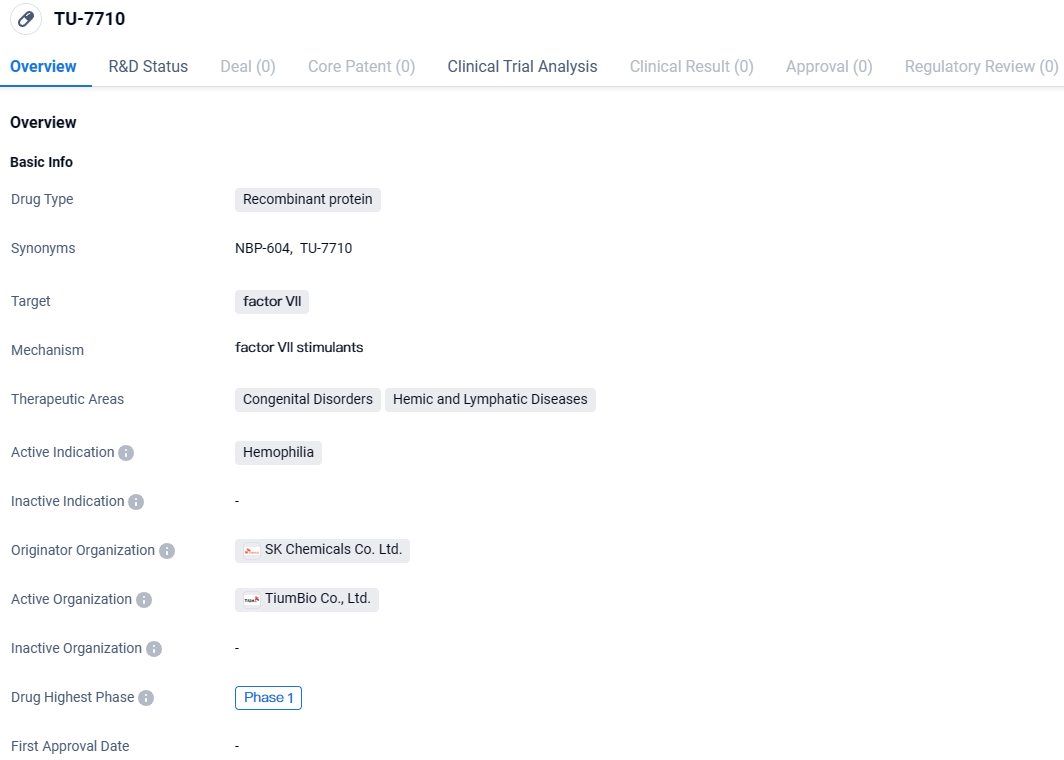
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The initial stage 1b medical study is a non-blind trial that includes both single and multiple ascending dosages intended to evaluate the safety profile, the capacity to be tolerated, the metabolic breakdown, and the therapeutic effects of the investigational drug TU7710. Its primary objective is to establish an optimal dosage for phase 2 trials. This study is set to recruit a maximum of 18 participants who are affected by factor VIII deficiency (hemophilia A) or factor IX deficiency (hemophilia B), all of whom have developed resistance to standard therapies. Research sites for this trial include various locations in both Italy and Spain.
TU7710 distinguishes itself as an extended-duration variant of recombinant Factor VIIa (rFVIIa), offering a substantial extension in biologic half-life—ranging from 6 to 7 times greater than that of the widely utilized NovoSeven, another format of rFVIIa. This is made possible through TiumBio's proprietary technology, which integrates a transferrin fusion mechanism. With these advancements, TU7710 is poised to significantly lower the frequency of doses required and the overall expense of treatment when compared to the administration of NovoSeven for those living with the condition.
Parallel to the aforementioned trial, TiumBio is actively pursuing a Phase 1a clinical study, concentrating on the assessment of TU7710's safety, degree of tolerance, pharmacokinetic and pharmacodynamic properties in a group of healthy adult male participants. We expect to disclose some preliminary findings from this ongoing research at the International Society on Thrombosis and Haemostasis (ISTH) gathering in 2024.
Hemophilia represents a hereditary pathology characterized by either an innate lack of critical coagulation factors or a reduction thereof, leading to significant bleeding tendencies. Two main types of this disorder are identified: hemophilia A, resulting from an insufficiency in coagulation factor VIII, and hemophilia B, resulting from a deficit in coagulation factor IX.
For patients diagnosed with hemophilia A or B who have developed inhibitory antibodies, rFVIIa serves as a compensatory therapy to bypass the conventional clotting cascade mechanisms. Nevertheless, therapeutic choices remain scarce in the current clinical landscape, with NovoSeven by Novo Nordisk being one of the few prominent treatments available to date.
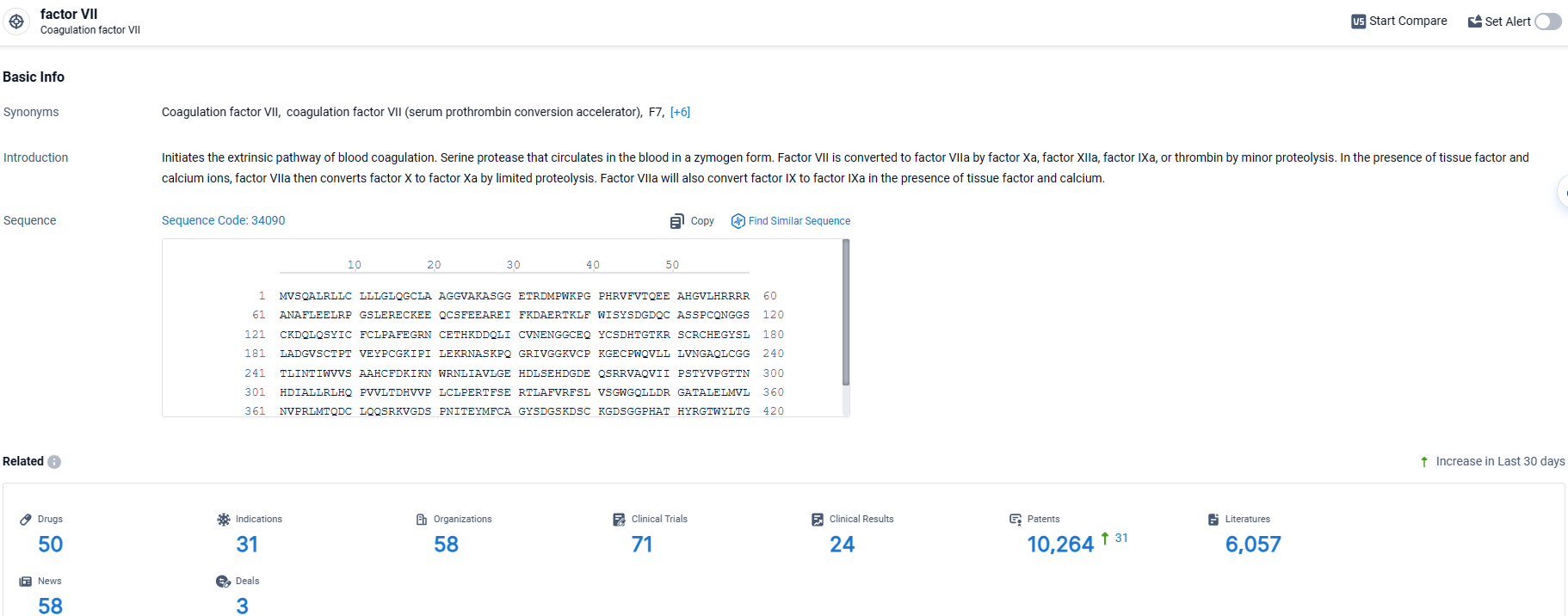
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 29, 2024, there are 50 investigational drugs for the factor VII target, including 31 indications, 58 R&D institutions involved, with related clinical trials reaching 71, and as many as 10264 patents.
TU-7710 targets factor VII and is intended for the treatment of hemophilia, a congenital disorder affecting blood clotting. Currently in Phase 1 of development, TU-7710 is being evaluated for its safety and potential efficacy. Further research and clinical trials will be necessary to determine the drug's effectiveness and potential impact on patients with hemophilia.