Understanding TNF Inhibitors and Methods to Keep Abreast of Their Recent Developments
Tumor Necrosis Factor (TNF) is a protein produced by immune cells in the human body. It plays a crucial role in regulating inflammation and immune responses. TNF acts as a signaling molecule, binding to specific receptors on cells and triggering a cascade of events that promote inflammation. It helps in the defense against infections and plays a role in the destruction of cancer cells. However, excessive or prolonged TNF activity can lead to chronic inflammation and contribute to various diseases, including autoimmune disorders like rheumatoid arthritis and inflammatory bowel disease. Pharmaceutical interventions targeting TNF have been developed to modulate its activity and treat these conditions.
The development of TNF inhibitors has been a significant advancement in the treatment of autoimmune diseases. The first TNF inhibitor on the world market was infliximab (Remicade), closely followed by etanercept (Enbrel), both in 1998. Adalimumab (Humira) followed in 2002. Insights gained from the development of TNF inhibitors have helped pave the way for the development of other anticytokine therapies.
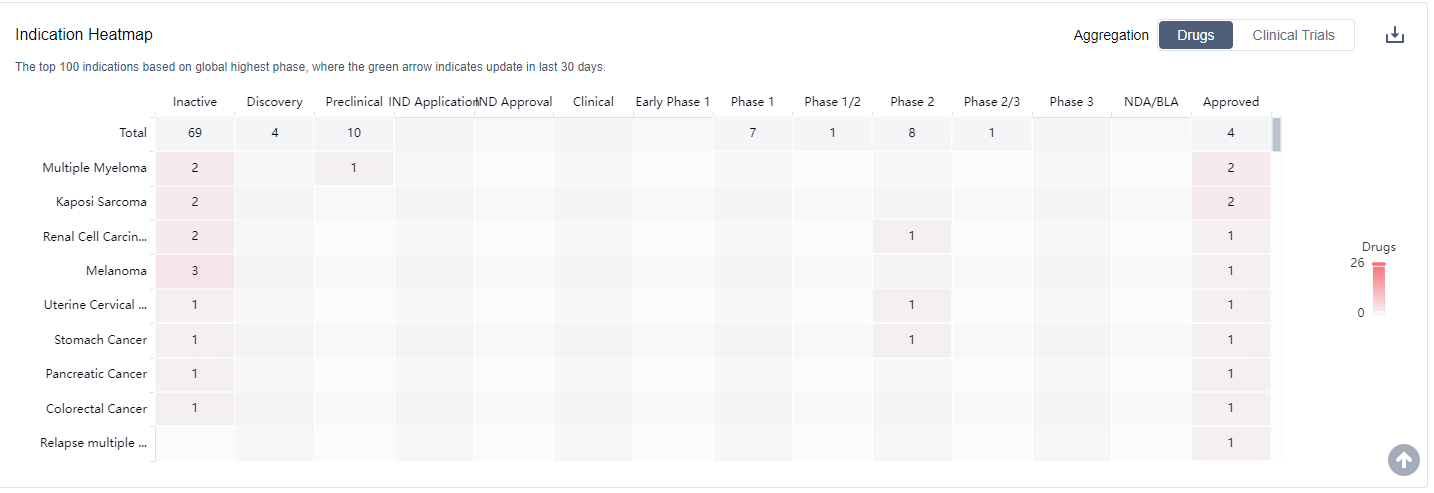
The analysis of the current competitive landscape and future development of target TNF reveals several key findings. Bristol Myers Squibb Co. is leading in terms of company growth and has the highest stage of development with two approved drugs. Multiple indications have been approved for drugs under the target TNF, indicating its potential in various therapeutic areas. The drug types progressing most rapidly include small molecule drugs, biosimilars, and other innovative drug types. The countries/locations with significant progress include Japan, United States, European Union, and China. China's progress in the development of target TNF drugs is noteworthy. Overall, the target TNF presents a competitive landscape with diverse companies, indications, drug types, and global development. Further analysis of R&D institutions, market dynamics, and regulatory factors will provide a more comprehensive understanding of the future prospects of target TNF in the pharmaceutical industry.
How do they work?
TNF inhibitors are a type of medication that target tumor necrosis factor (TNF), a protein involved in the inflammatory response of the immune system. From a biomedical perspective, TNF inhibitors are used in the treatment of various autoimmune diseases, such as rheumatoid arthritis, psoriasis, Crohn's disease, and ankylosing spondylitis. These inhibitors work by blocking the action of TNF, thereby reducing inflammation and alleviating symptoms associated with these conditions. By suppressing the immune response, TNF inhibitors help to control the overactive immune system and prevent further damage to tissues and joints.
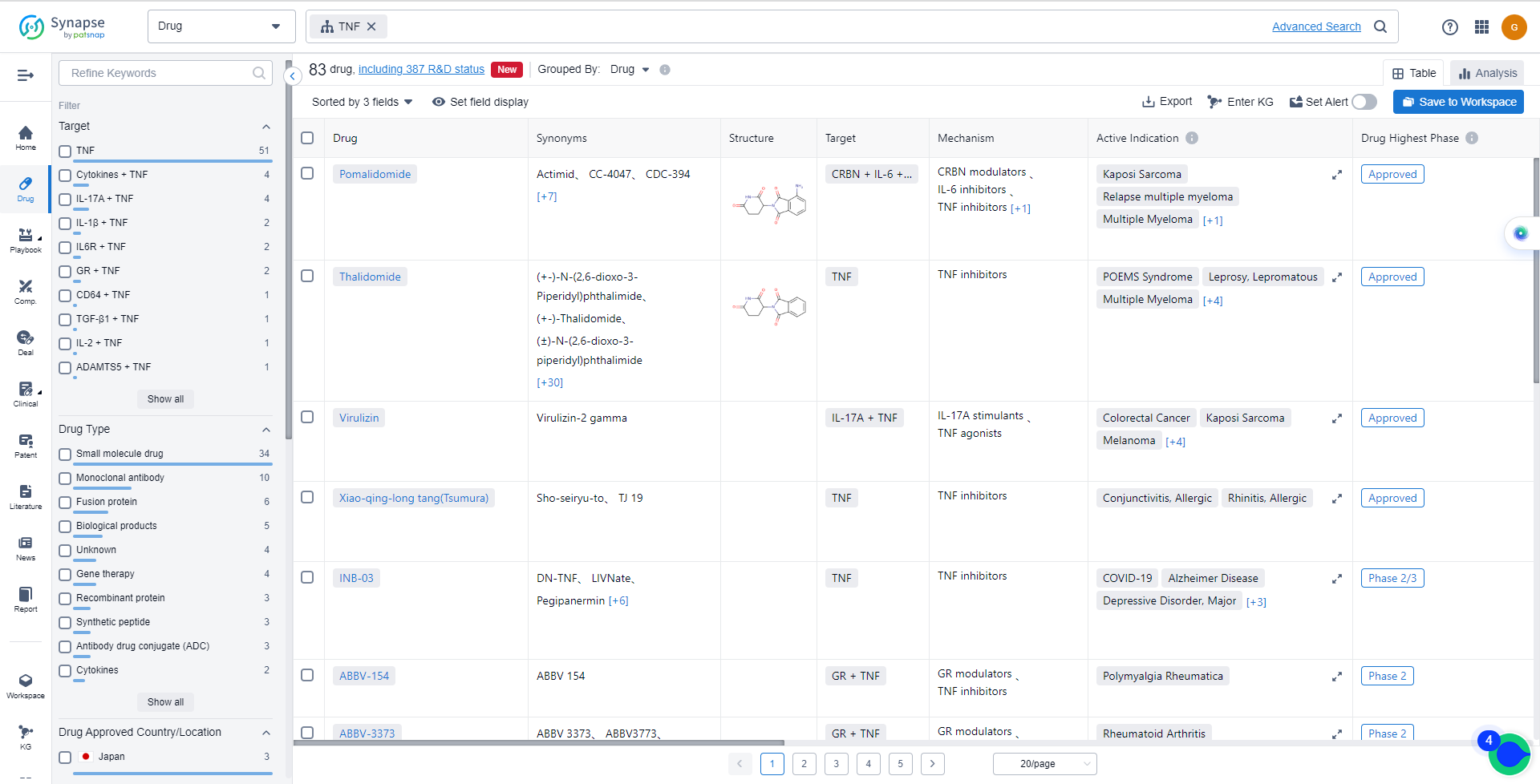
List of TNF Inhibitors
The currently marketed TNF inhibitors include:
- Pomalidomide
- Thalidomide
- Virulizin
- Xiao-qing-long tang(Tsumura)
- INB-03
- ABBV-154
- ABBV-3373
- Leramistat
- OC-001
- SAR-441566
For more information, please click on the image below.
What are TNF inhibitors used for?
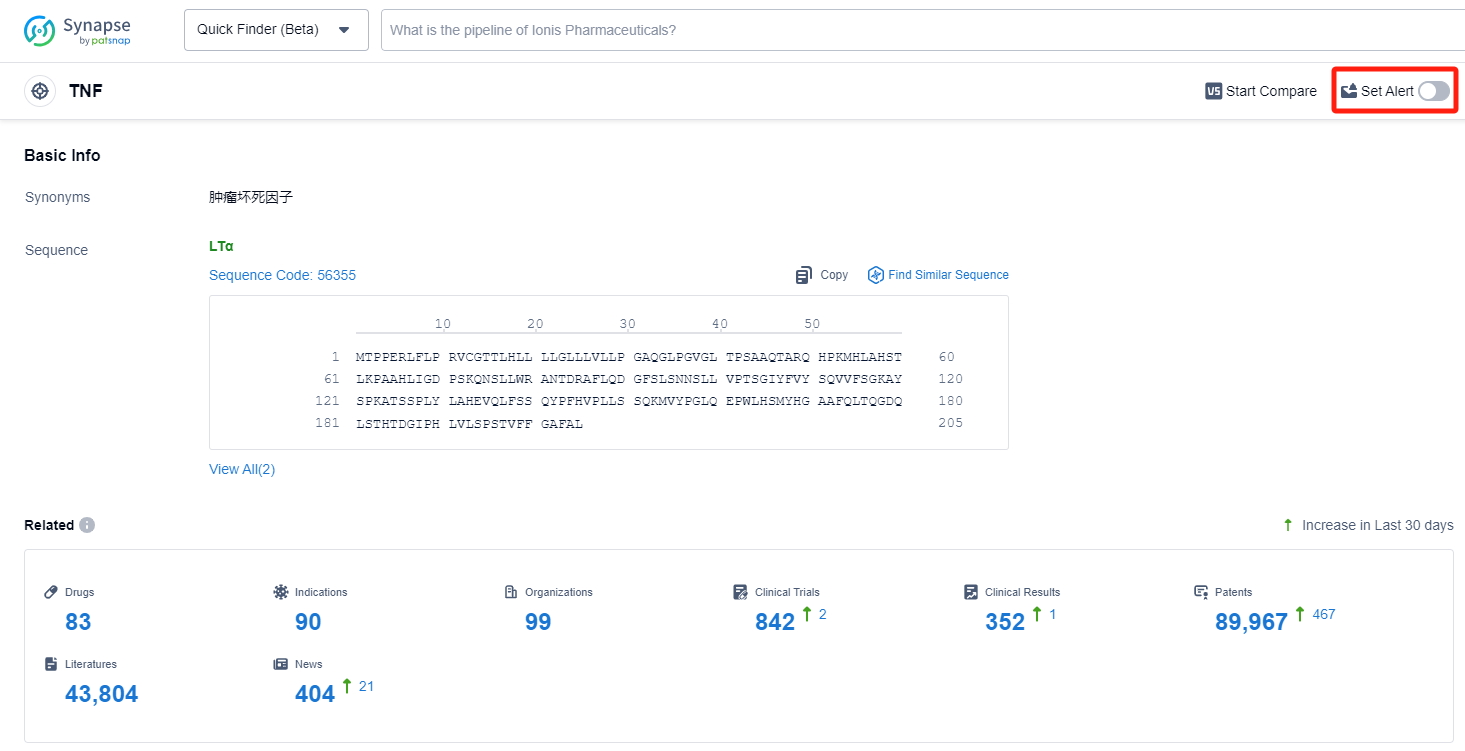
TNF inhibitors are used in the treatment of various autoimmune diseases, such as rheumatoid arthritis, psoriasis, Crohn's disease, and ankylosing spondylitis. For more information, please click on the image below to log in and search.
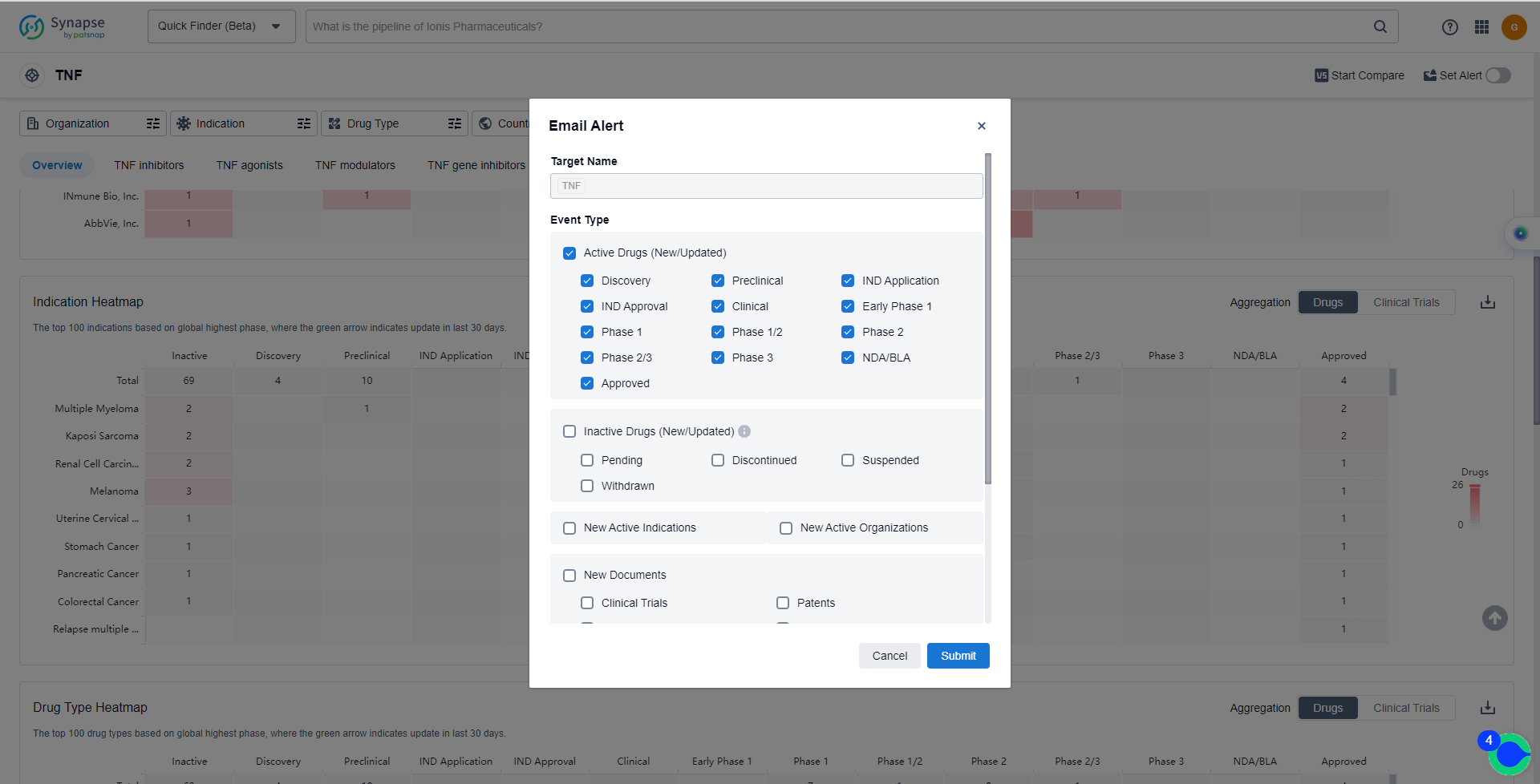
How to obtain the latest development progress of TNF inhibitors?
In the Synapse database, you can keep abreast of the latest research and development advances of TNF inhibitors anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!