Trehalose: Detailed Review on its Transformative R&D Success, Mechanism of Action, and Drug Targets
Trehalose's R&D Progress
Trehalose is a small molecule drug that targets the transcription factor EB (TFEB). It is being developed for the treatment of various therapeutic areas including Nervous System Diseases, Congenital Disorders, Endocrinology and Metabolic Diseases, and Skin and Musculoskeletal Diseases. The drug has shown potential in treating specific indications such as Machado-Joseph Disease, Muscular Dystrophy, Oculopharyngeal, Spinocerebellar Ataxias, Amyotrophic Lateral Sclerosis, Bulbo-Spinal Atrophy, and X-linked disorders.
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: The originator organization of Trehalose is Bioblast Pharma Ltd. The drug has reached the highest phase of clinical development, which is Phase 3 globally.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In terms of regulation, Trehalose has received designations such as Rare Pediatric Disease, Fast Track, and Orphan Drug. These designations are granted by regulatory authorities to drugs that target rare diseases or conditions with unmet medical needs. The Rare Pediatric Disease designation highlights the drug's potential to address diseases affecting children, while the Fast Track designation expedites the development and review process for drugs that demonstrate the potential to address significant unmet medical needs. The Orphan Drug designation provides incentives to encourage the development of drugs for rare diseases.
Mechanism of Action for Trehalose: TFEB activators
TFEB activators are substances or compounds that stimulate the activity of the transcription factor EB (TFEB). TFEB is a protein that plays a crucial role in regulating cellular processes such as autophagy, lysosomal biogenesis, and lipid metabolism. It acts as a master regulator of the cellular response to stress and nutrient availability.
From a biomedical perspective, TFEB activators can be of great interest in the field of biomedicine. By activating TFEB, these compounds have the potential to enhance cellular clearance mechanisms, promote the degradation of toxic proteins, and improve cellular health.
TFEB activators can be small molecules or natural compounds that directly bind to TFEB or modulate its activity indirectly through signaling pathways. They can promote the translocation of TFEB from the cytoplasm to the nucleus, where it can activate the expression of genes involved in autophagy and lysosomal function.
Overall, TFEB activators have the potential to be therapeutic agents for various diseases by enhancing cellular clearance mechanisms and promoting cellular health. However, further research is needed to fully understand their mechanisms of action and evaluate their efficacy and safety in clinical settings.
Drug Target R&D Development for Trehalose
The drug target of Trehalose is TFEB. TFEB is a crucial regulator of cellular processes in the human body. It plays a significant role in the clearance of cellular waste and the maintenance of cellular homeostasis. TFEB controls the expression of genes involved in autophagy, lysosomal biogenesis, and lipid metabolism. By activating these pathways, TFEB promotes the removal of damaged organelles and proteins, thereby preventing the accumulation of toxic substances. Additionally, TFEB has been linked to various diseases, including neurodegenerative disorders and cancer, making it an attractive target for therapeutic interventions in the pharmaceutical industry.
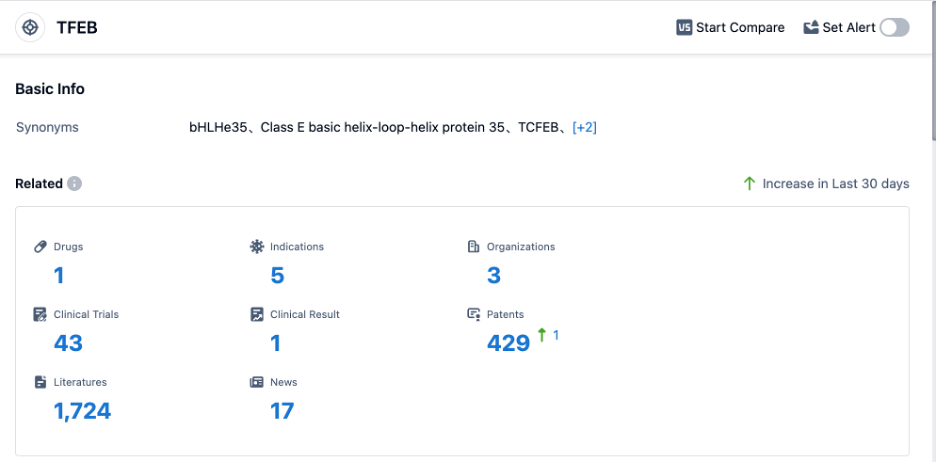
According to PatSnap Synapse, as of 30 Aug 2023, there are a total of 1 TFEB drug worldwide, from 3 organizations, covering 5 indications, and conducting 43 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
Conclusion
In summary, Trehalose is a small-molecule drug developed by Bioblast Pharma Ltd. It targets TFEB and is being investigated for the treatment of various therapeutic areas, including Nervous System Diseases, Congenital Disorders, Endocrinology and Metabolic Diseases, and Skin and Musculoskeletal Diseases. The drug has shown promise in treating specific indications such as Machado-Joseph Disease, Muscular Dystrophy, Oculopharyngeal, Spinocerebellar Ataxias, Amyotrophic Lateral Sclerosis, Bulbo-Spinal Atrophy, and X-linked disorders. Trehalose has reached Phase 3 of clinical development globally, indicating advanced stages of testing. It has also received regulatory designations such as Rare Pediatric Disease, Fast Track, and Orphan Drug, highlighting its potential to address rare diseases and unmet medical needs.