TROPION-Lung01 Trial: Datopotamab Deruxtecan Improves Survival in Advanced Non-Small Cell Lung Cancer
The top-line overall survival outcomes from the TROPION-Lung01 Phase III study, which earlier achieved the dual primary endpoint of progression-free survival, showed a numerical advantage for datopotamab deruxtecan (Dato-DXd) over docetaxel in the complete cohort of patients with locally advanced or metastatic non-small cell lung cancer who had received at least one previous treatment.
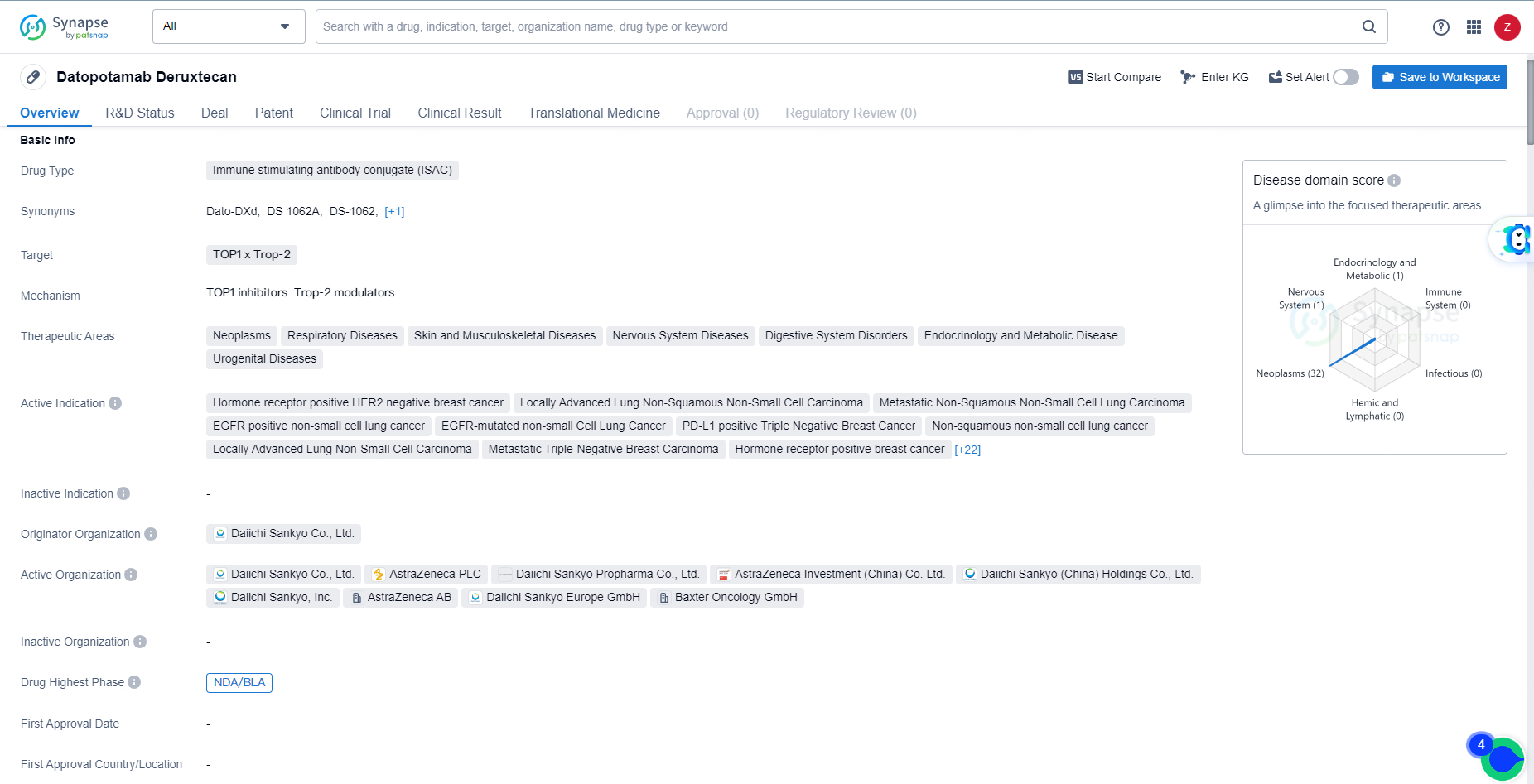
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Survival outcomes did not achieve statistical significance across the entire trial population. However, in the predefined subgroup of patients with nonsquamous non-small cell lung cancer (NSCLC), datopotamab deruxtecan demonstrated a clinically meaningful improvement in overall survival (OS) compared to docetaxel, the current standard chemotherapy.
The final OS analysis extends the positive progression-free survival (PFS) results presented at the 2023 European Society for Medical Oncology Congress, where datopotamab deruxtecan showed a statistically significant PFS benefit for the entire trial population and a clinically meaningful PFS benefit for patients with nonsquamous NSCLC. In the TROPION-Lung01 study, patient enrollment by tumor histology was balanced across treatment groups and aligned with real-world incidence rates, with approximately 75% of patients having nonsquamous NSCLC.
The safety profile of datopotamab deruxtecan in TROPION-Lung01 was consistent with previous analyses, showing fewer dose reductions or treatment discontinuations due to adverse events compared to docetaxel, and no new safety concerns were identified. No new interstitial lung disease events of any grade were determined to be drug-related.
Susan Galbraith, Executive Vice President of Oncology R&D at AstraZeneca, stated: “Datopotamab deruxtecan is the only investigational therapy that has shown a clinically meaningful survival improvement in previously treated patients with nonsquamous non-small cell lung cancer versus docetaxel, which has long remained unmatched in this post-targeted treatment and post-immunotherapy setting. These results highlight the potential for datopotamab deruxtecan to replace traditional chemotherapy in this advanced treatment setting and emphasize our confidence in ongoing trials evaluating this therapy for first-line lung cancer.”
Ken Takeshita, MD, Global Head of R&D at Daiichi Sankyo, commented: “The observed improvement in overall survival with datopotamab deruxtecan, along with the previously reported clinically meaningful PFS, more than doubling of overall response, and prolonged response duration compared to docetaxel, suggests that this TROP2-directed antibody drug conjugate could emerge as a significant new treatment for patients with advanced nonsquamous non-small cell lung cancer.”
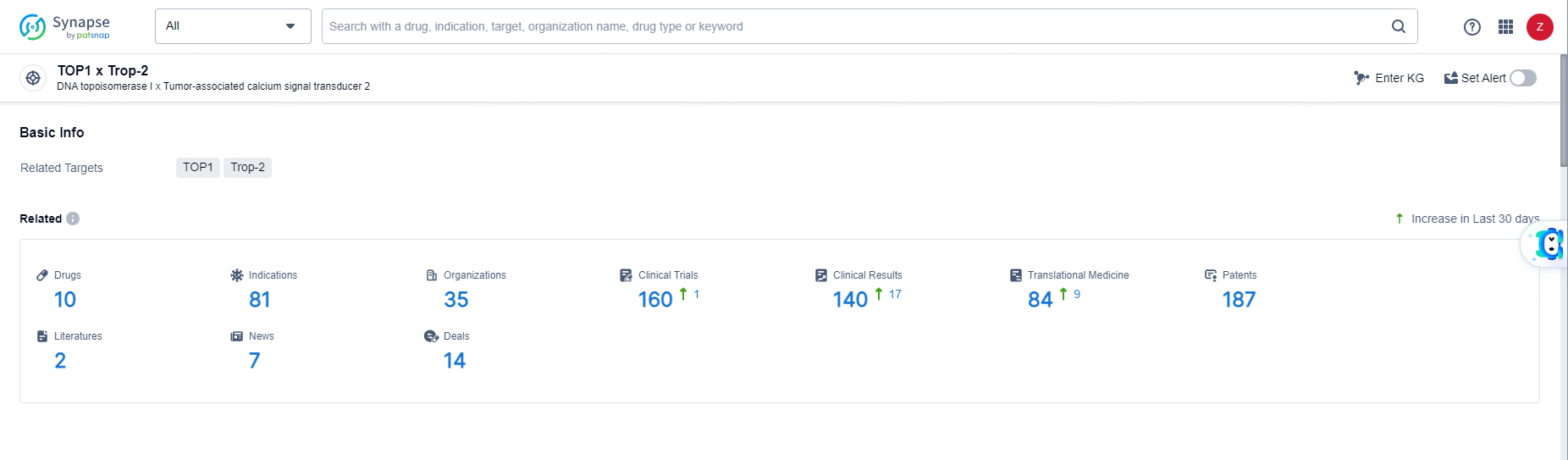
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 31, 2024, there are 10 investigational drugs for the TOP1 and Trop2 targets, including 81 indications, 35 R&D institutions involved, with related clinical trials reaching 160, and as many as 187 patents.
Datopotamab Deruxtecan is an ISAC drug with a broad therapeutic scope, targeting various types of cancers. It is developed by Daiichi Sankyo Co., Ltd. and has advanced to the NDA/BLA phase in both global and Chinese markets. The drug's potential to address a wide range of therapeutic areas and indications makes it a significant candidate in the field of biomedicine and pharmaceuticals.