GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (2)
This article will continue to introduce the GPCR (G Protein-Coupled Receptor) targets and pipelines that garnered attention for AstraZeneca, Pfizer, and Eli Lilly at the JP Morgan Healthcare Conference 2024.For more details, please follow the link below.
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (1)
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (3)
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (4)
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (5)
AstraZeneca in JPM 2024
AstraZeneca, as a science-led global biopharmaceutical company, focuses on five major disease areas: oncology, rare diseases, cardiovascular/renal and metabolic diseases, respiratory and immunology, as well as vaccines and immunotherapy. In the first nine months of 2023, the company generated total revenues of $33.8 billion.
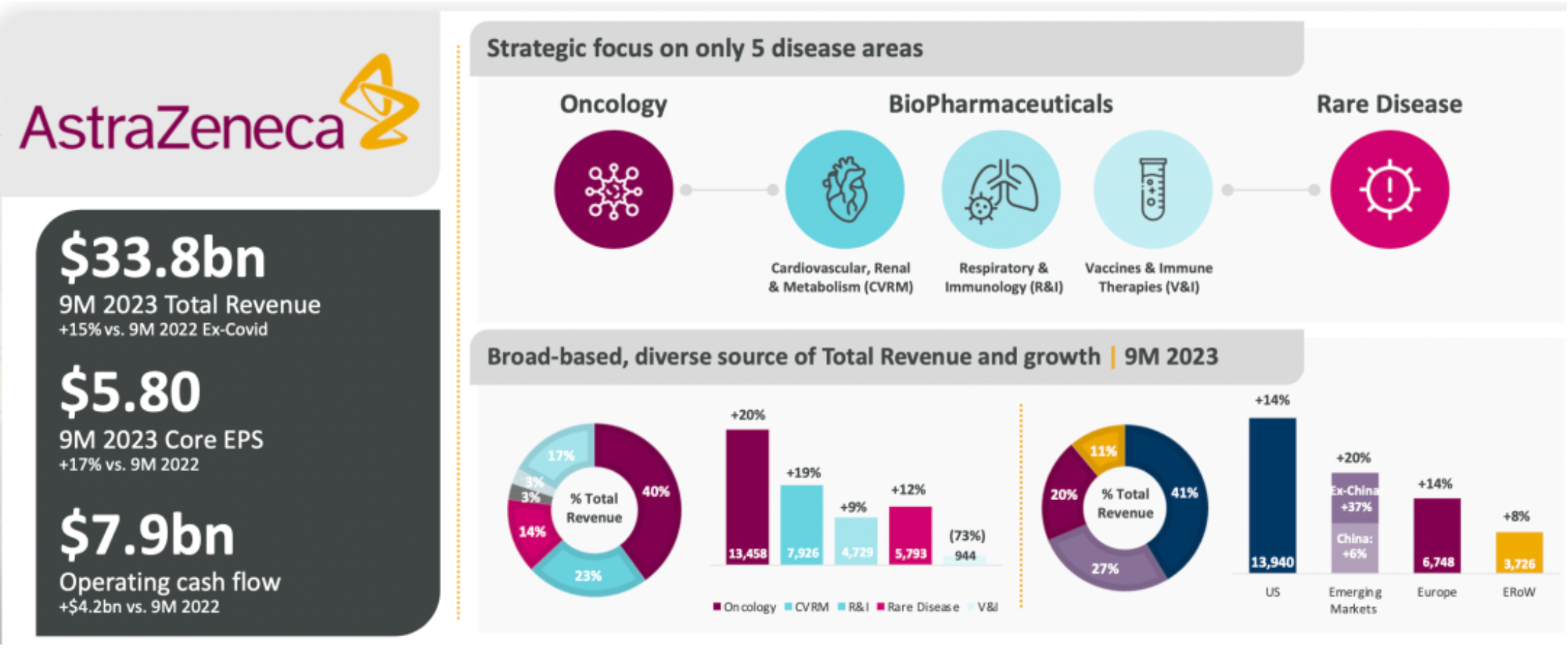
The company will continue to concentrate on clinical development, advancing its leadership position in disease areas, and extensively exploring its promising, diverse product portfolio. Meanwhile, the company's business development momentum is strong, having bolstered its leading position in disease areas and its next-wave capabilities through value-adding transactions, including the acquisition of the oral GLP-1R agonist project from Eccogene.
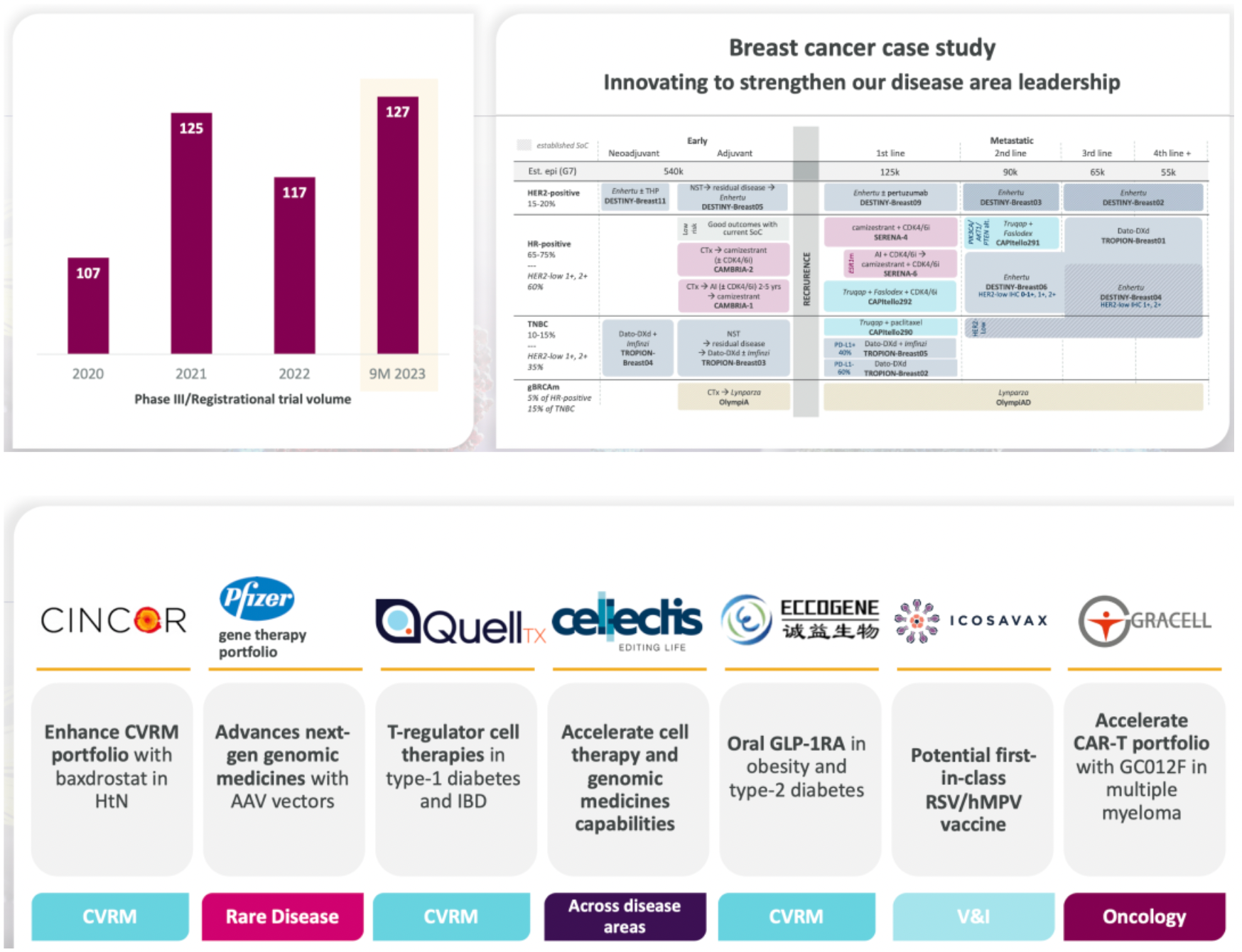
LM-305 is a monoclonal antibody ADC product targeting the GPRC5D receptor, developed by Lixian Pharmaceuticals. On May 12, 2023, LaNova Medicines announced the signing of an exclusive licensing agreement with AstraZeneca for LM-305, a pre-clinical stage antibody-drug conjugate comprising an anti-GPRC5D monoclonal antibody, a degradable protease linker, and a cytotoxic payload of MMAE.
Zibotentan is an antagonist targeting the ETA receptor and is currently undergoing Phase 2 clinical development for the treatment of portal hypertension, cirrhosis, albuminuria, type 2 diabetes, chronic kidney disease, liver disease, and more. In addition, a combination product of Zibotentan and Dapagliflozin (an SGLT2 inhibitor), is undergoing Phase 3 clinical studies for chronic kidney disease, proteinuria, cirrhosis, etc.
Breztri Aerosphere (Budesonide/Formoterol Fumarate/Glycopyrronium Bromide) is a combination small molecule formulation that works as a glucocorticoid receptor agonist, mAChRs antagonist, and β2AR agonist, intended for the treatment of chronic obstructive pulmonary disease (COPD).
AZD9550, developed by AstraZeneca, is a dual agonist for the GCGR/GLP-1R receptors. Additionally, AZD5004 (ECC5004) is an exclusive licensed product from a collaboration between AstraZeneca and Eccogene, ECC5004 being an investigational oral once-daily GLP-1RA agonist designed for the treatment of obesity, type 2 diabetes, and other cardiac metabolic diseases.
AZD5462, an original development by AstraZeneca, is a relaxin receptor RXFP1 agonist for the treatment of heart failure and is currently in Phase 1 clinical studies. Preclinical studies in monkey models with heart failure with reduced ejection fraction (HFrEF) have shown an improvement in cardiac contractility after 8 weeks of treatment, without a significant increase in mean arterial blood pressure or heart rate. AZD5462 has high selectivity for RXFP1 and lacks activity against several other closely related GPCRs.
AZD6234, an original development by AstraZeneca, is a long-acting insulin receptor agonist and CALCR receptor agonist intended for clinical development in the treatment of obesity.
Pfizer in JPM 2024
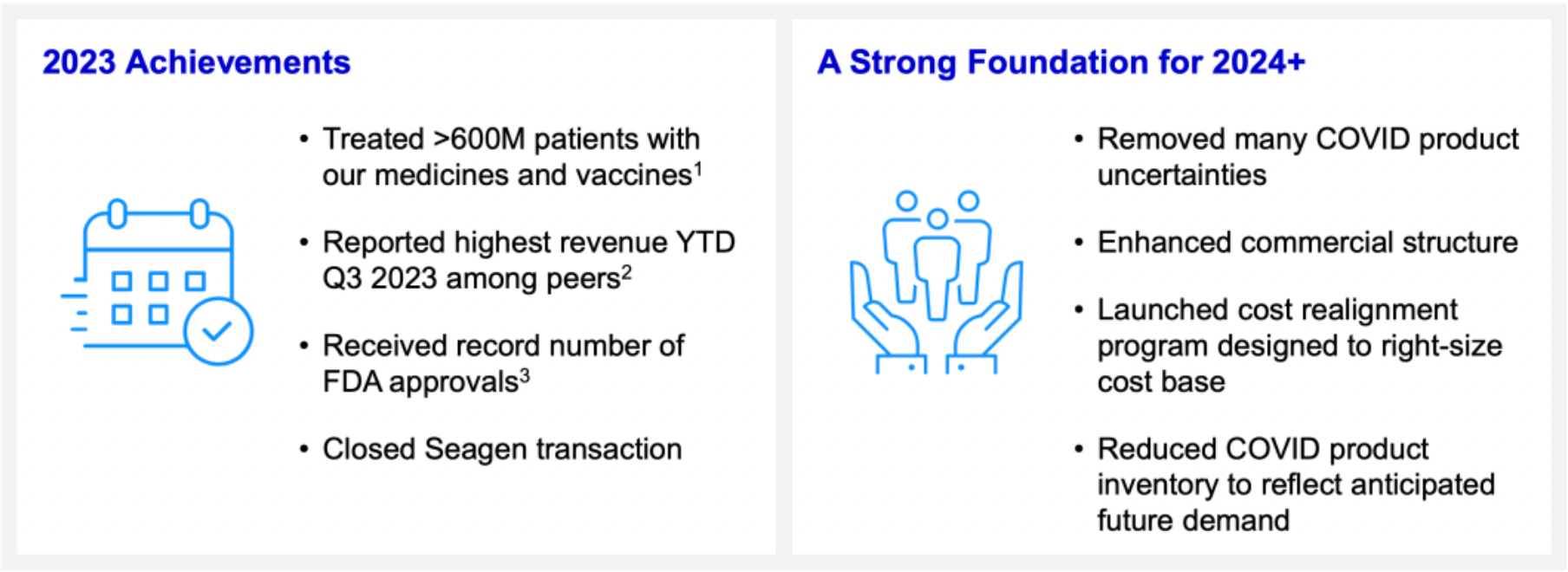
In 2023, Pfizer provided medication and vaccine treatments to over 600 million patients worldwide. By the third quarter, the company achieved the highest revenue record among its peers. During this year, Pfizer received a record number of FDA New Molecular Entity approvals, including products such as Zavzpret, Paxlovid, Abrysvo (for OA+MI), Litfulo, Ngenla, Elrexfio, Comirnaty, Velsipity, and Penbraya.
Velsipity (Etrasimod) is used for the treatment of moderate to severe ulcerative colitis. It is a selective sphingosine-1-phosphate receptor modulator S1PR1+S1PR4+S1PR5, which was added to Pfizer’s portfolio during the acquisition of Arena Pharmaceuticals in 2022.
Zavzpret (zavegepant) is a CGRP receptor antagonist. Zavzpret is both the world's first and only nasal spray formulation of a calcitonin gene-related peptide (CGRP) receptor antagonist. On March 9, Pfizer’s Zavzpret entered a crowded migraine treatment market.
Eli Lilly in JPM 2024
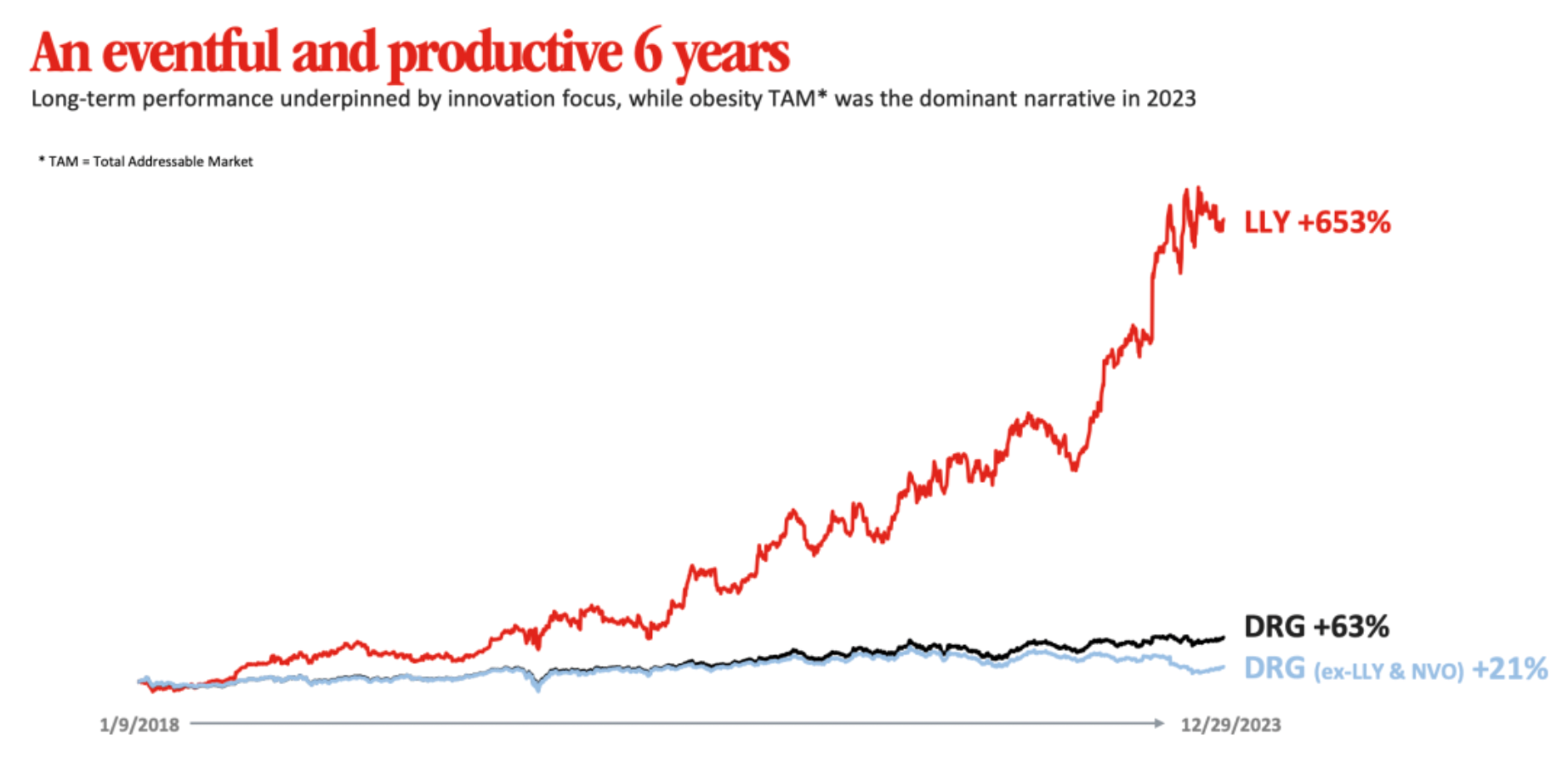
Over the past six years, Eli Lilly has experienced a stock price increase of over 600%, making it the biopharmaceutical company with the highest market value in the world. Innovation serves as the foundation for sustainable performance, and obesity is the main theme for the year 2023.
The company focuses on four major research areas: diabetes, obesity and cardiovascular metabolism, cancer, neurodegenerative diseases, and immunological disorders. It has made substantial investments in various therapeutic domains, such as gene therapies, to accelerate the pace of research and development.
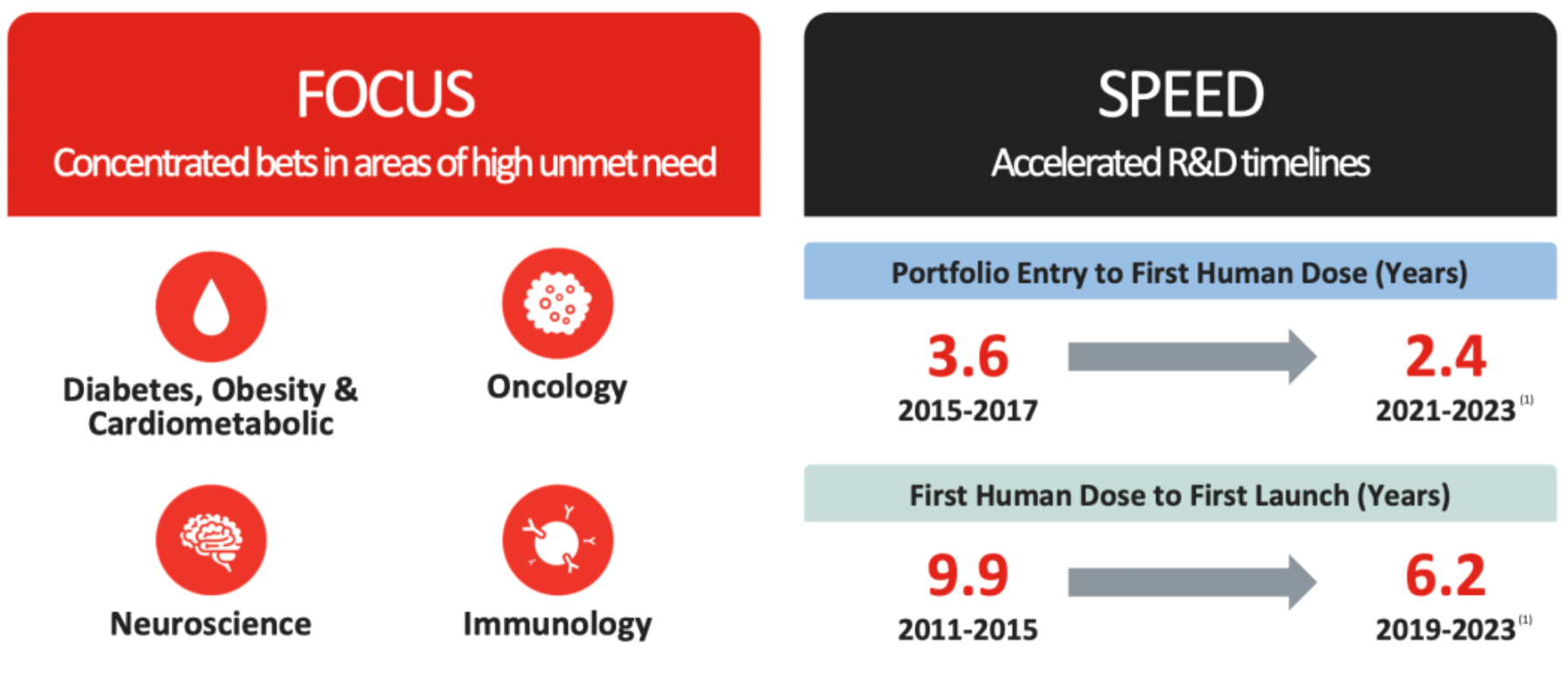
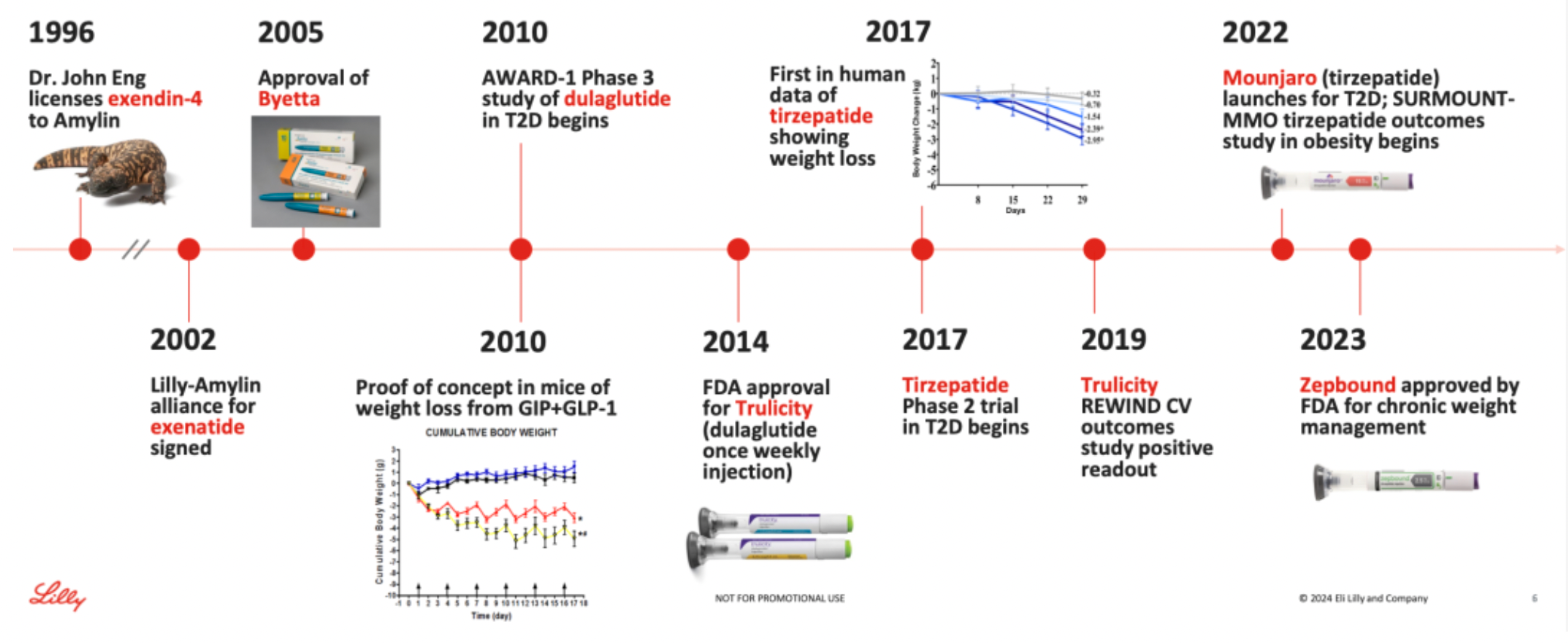
Success is never a matter of chance; it results from sustained and continuous research and development investment over an extended period of time. In the field of incretin-related therapies, the company has persevered in research and development efforts for over 30 years.
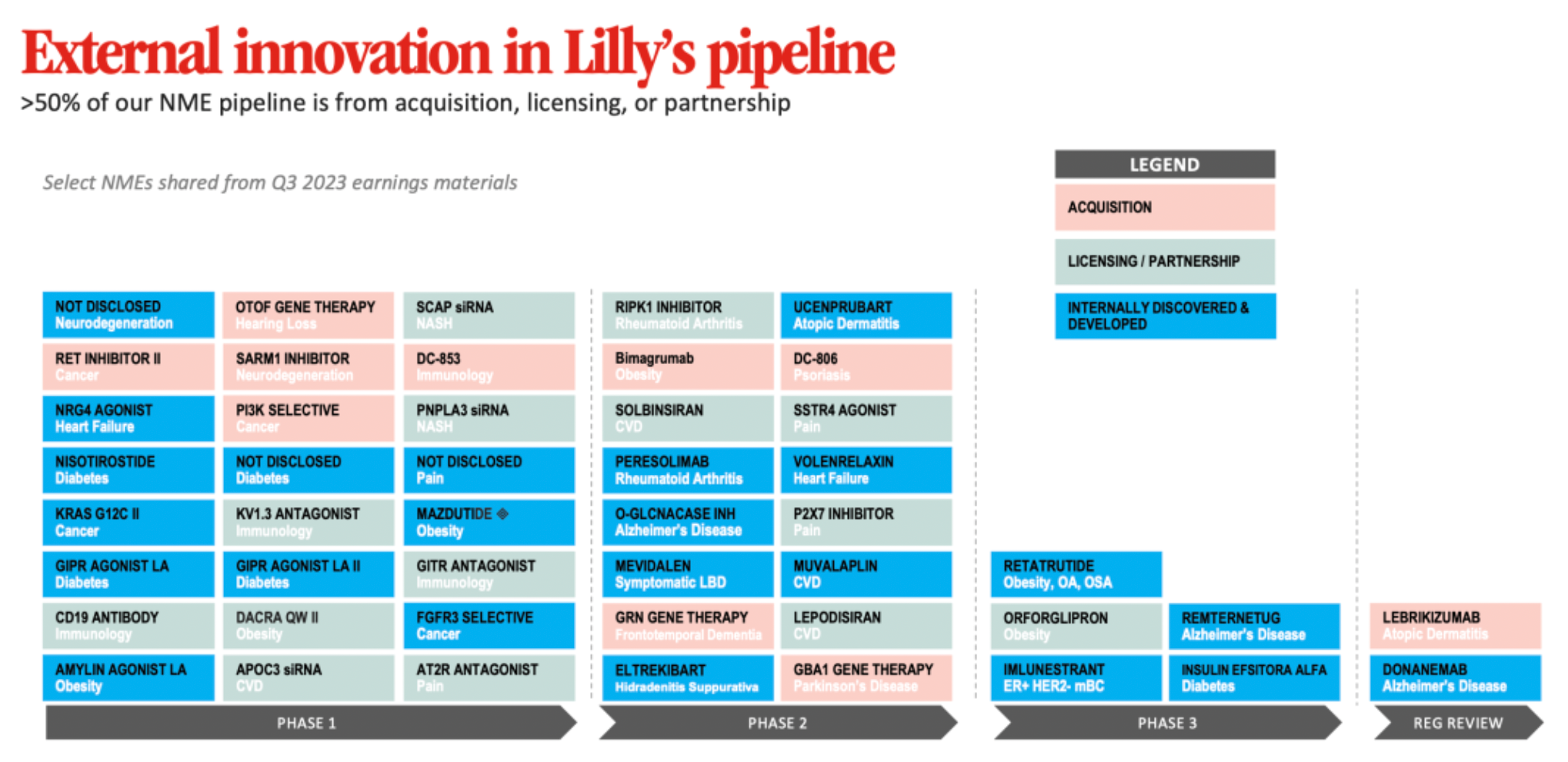
External innovation is extremely important to Eli Lilly and Company, with over 50% of the company's new drug development pipeline coming from acquisitions, licensing, or collaboration.
Retatrutide is a GIPR/GLP-1R/GCGR triple agonist originally developed by Eli Lilly and Company. On May 22, Eli Lilly formally submitted an application for a randomized, double-blind, phase 3 clinical study of the GIP/GLP-1/Glucagon triple agonist Retatrutide (LY3437943). The product is currently in the phase 3 clinical study stage. (Extended reading on Retatrutide: Significant: Eli Lilly will announce the phase 2 clinical trial results of the triple agonist Retatrutide (LY3437943) at ADA 2023; Eli Lilly releases clinical data on the triple agonist Retatrutide, with fierce competition in the GLP-1R agonist market!)
Orforglipron (OWL833, LY3502970) is a small molecule agonist targeting GLP-1R, originally developed by Chugai. In 2018, Eli Lilly entered into a collaboration agreement with Chugai and paid a $50 million upfront payment to obtain the rights to clinically develop Orforglipron for the treatment of type 2 diabetes. (Extended reading: After Tirzepatide's approval for the treatment of obesity, how long will it be before Eli Lilly's small molecule GLP-1R agonist becomes available?)
Volenrelaxin (LY3540378) is a long-acting synthetic relaxin analogue that acts as an agonist for the relaxin family peptide receptor 1 (RXFP1), developed by Eli Lilly for the treatment of chronic heart failure. It is currently in phase 2 clinical trials.
LY3556050 is a small molecule SSTR4 agonist designed to modulate somatostatin signaling, affecting neuroexcitability and thus improving the regulation of inflammation and pain. It is currently undergoing phase 2 clinical development for the treatment of pain.
LY3154207 (Mevidalen) is a small molecule compound that acts as a positive allosteric modulator of the dopamine receptor D1R. It is currently undergoing phase 2 clinical studies for the treatment of symptomatic leukoencephalopathy.
LY3041658 (Eltrekibart) is a neutralizing chemokine that specifically blocks the activity of the CXCR1 and CXCR2 receptors. A phase 2 clinical study is currently underway for LY3041658 to treat pyoderma gangrenosum eczema.
CFTX-1554 is a novel inhibitor targeting the GPCR family member Angiotensin II type 2 receptor (AT2R). CFTX-1554 was originally developed by Confo Therapeutics, and in May of the year '23, Eli Lilly reached a global licensing agreement with Confo for CFTX-1554 and other candidate compounds. CFTX-1554 is currently in phase 1 clinical studies for the development of treating neuropathic pain.
Mazdutide is a synthetic peptide that acts as a GCGR+GLP-1R dual agonist, currently being developed in China in collaboration with Sinopharm for the treatment of type 2 diabetes and obesity.