What are PDE4 inhibitors and how do you quickly get the latest development progress?
Phosphodiesterase 4 (PDE4) is an enzyme found in various tissues of the human body, including the immune cells, brain, and lungs. It plays a crucial role in regulating the levels of cyclic adenosine monophosphate (cAMP), a signaling molecule involved in numerous cellular processes. PDE4 specifically breaks down cAMP, thereby controlling its concentration and influencing various physiological functions. In the immune system, PDE4 inhibition can reduce the production of pro-inflammatory cytokines, making it a target for anti-inflammatory drugs. Additionally, PDE4 inhibitors have shown potential in treating respiratory diseases like asthma and chronic obstructive pulmonary disease (COPD) by relaxing airway smooth muscles.
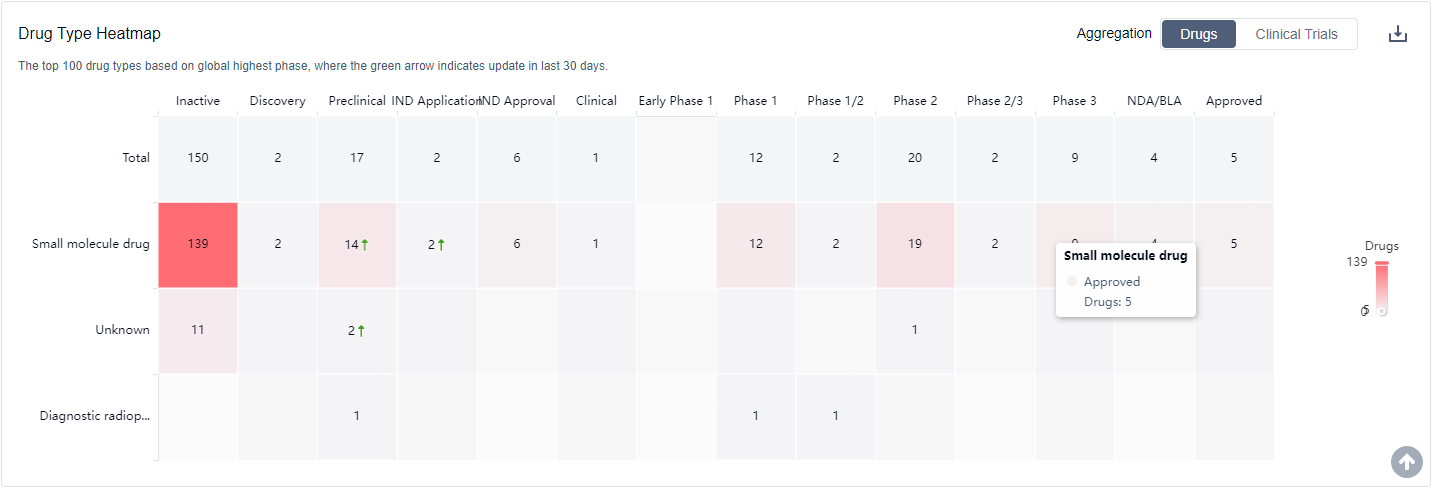
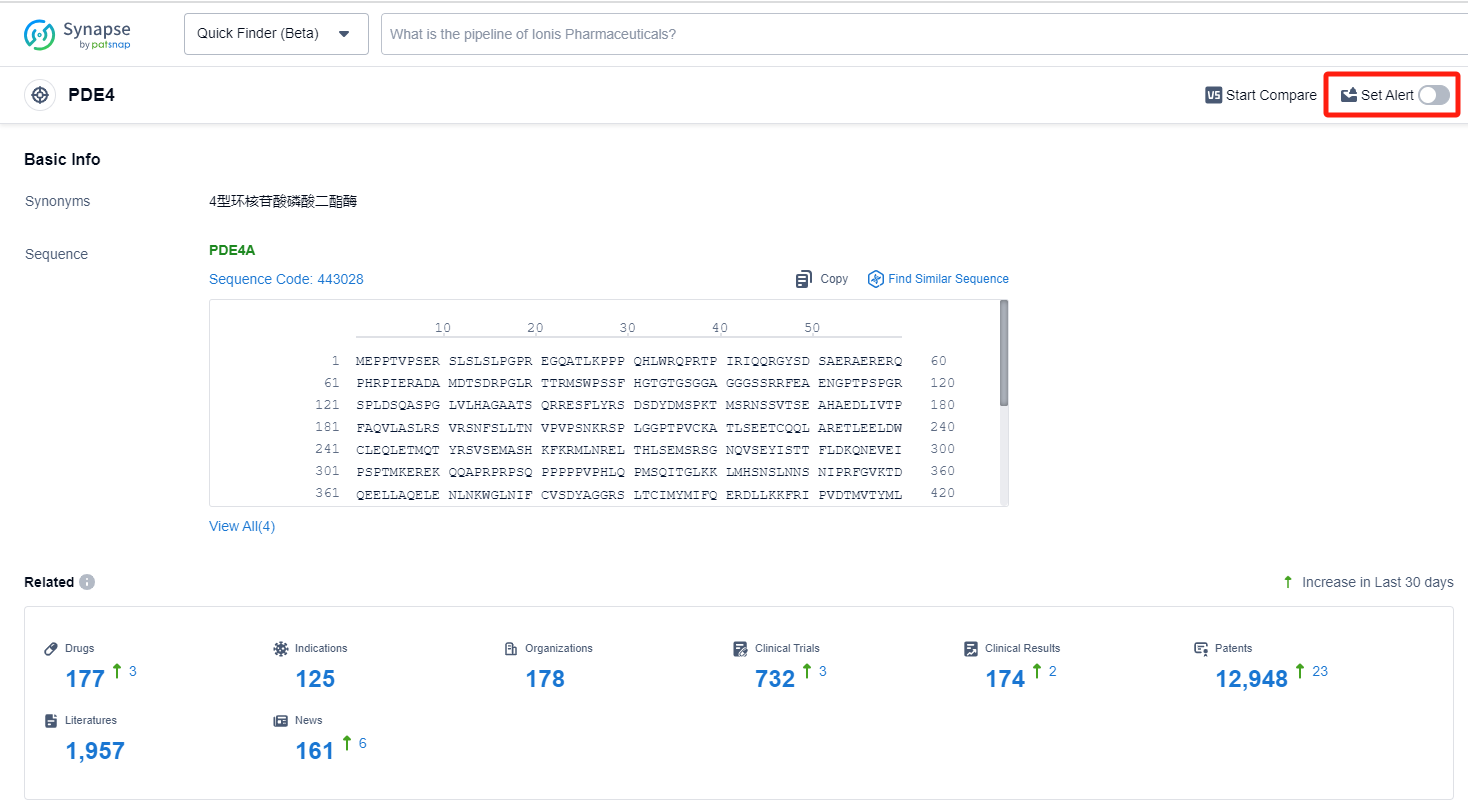
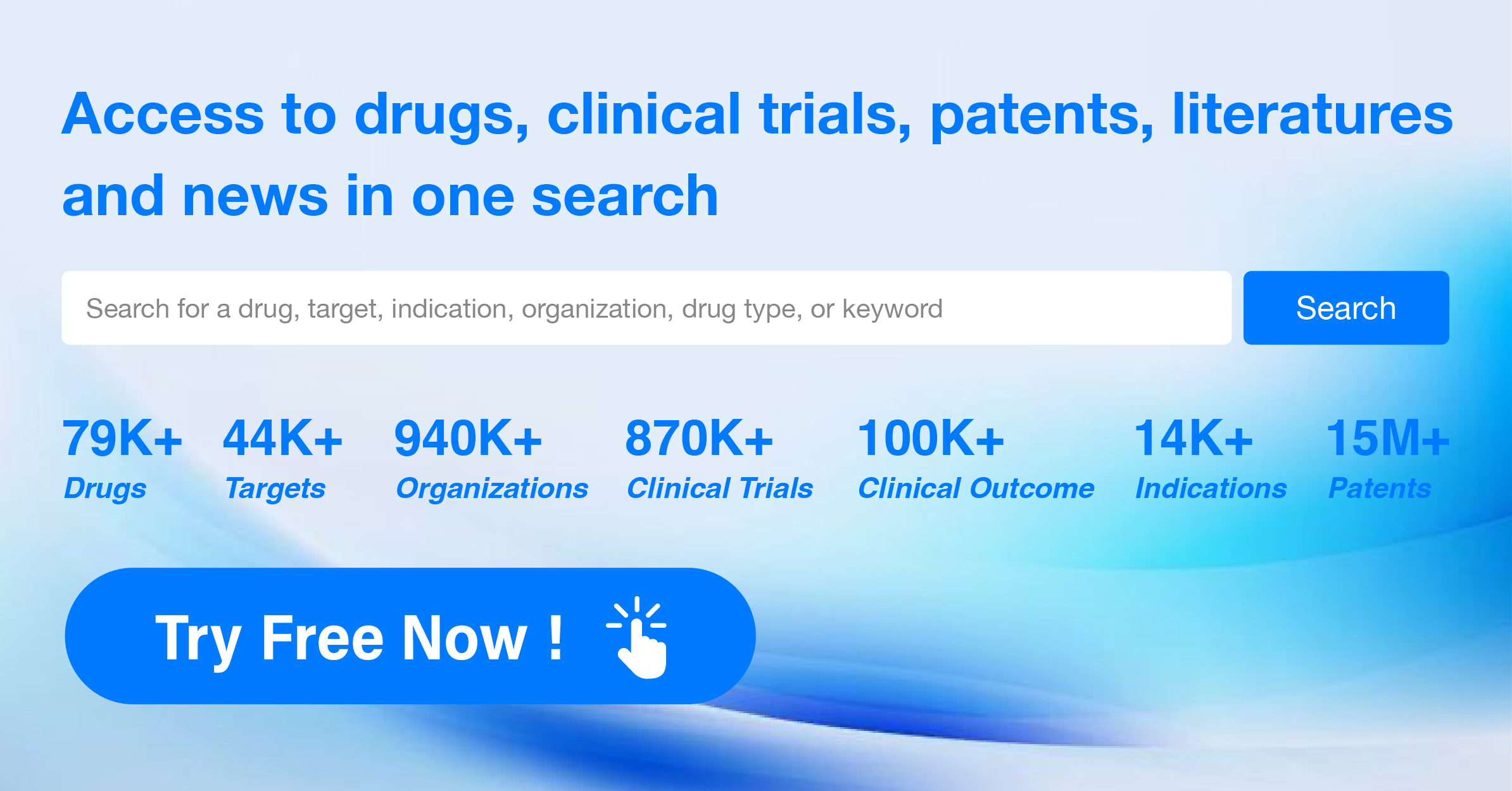
The analysis of the target PDE4 in the pharmaceutical industry reveals a competitive landscape with multiple companies actively involved in research and development. Pfizer Inc. stands out with the highest stage of development and a significant number of drugs in various phases. Plaque psoriasis, Asthma, and Pulmonary Disease, Chronic Obstructive are the indications with the highest number of approved drugs. Small molecule drugs and Unknown drugs, including biosimilars, are progressing rapidly. China, the United States, and the European Union are the leading countries/locations in drug development under the target PDE4.
The future development of target PDE4 will depend on the success of ongoing clinical trials, the ability to overcome challenges in drug development, and the competitive landscape among companies and countries/locations. Further analysis of individual companies' R&D strategies, indication-specific developments, and the impact of biosimilars will provide a more comprehensive understanding of the future prospects for target PDE4 in the pharmaceutical industry.
How do they work?
PDE4 inhibitors are a type of drug that specifically targets and inhibits the enzyme phosphodiesterase 4 (PDE4). Phosphodiesterase enzymes are responsible for breaking down cyclic adenosine monophosphate (cAMP), a molecule involved in cell signaling. By inhibiting PDE4, these inhibitors increase the levels of cAMP in cells.
From a biomedical perspective, PDE4 inhibitors have been primarily studied and used in the treatment of inflammatory conditions such as asthma, chronic obstructive pulmonary disease (COPD), and psoriasis. In these conditions, excessive inflammation plays a key role, and PDE4 inhibitors help to reduce inflammation by modulating the immune response.
PDE4 inhibitors work by blocking the action of PDE4 enzyme, which in turn prevents the breakdown of cAMP. Increased levels of cAMP lead to various anti-inflammatory effects, such as reducing the production of pro-inflammatory cytokines and chemokines, inhibiting the activation of immune cells, and promoting bronchodilation in the airways.
Some examples of PDE4 inhibitors include roflumilast, apremilast, and cilomilast. These drugs are typically administered orally and have shown efficacy in reducing inflammation and improving symptoms in patients with inflammatory conditions.
It is important to note that PDE4 inhibitors may have side effects, including gastrointestinal disturbances, headache, and in some cases, psychiatric symptoms. Therefore, their use should be carefully monitored and prescribed by healthcare professionals.
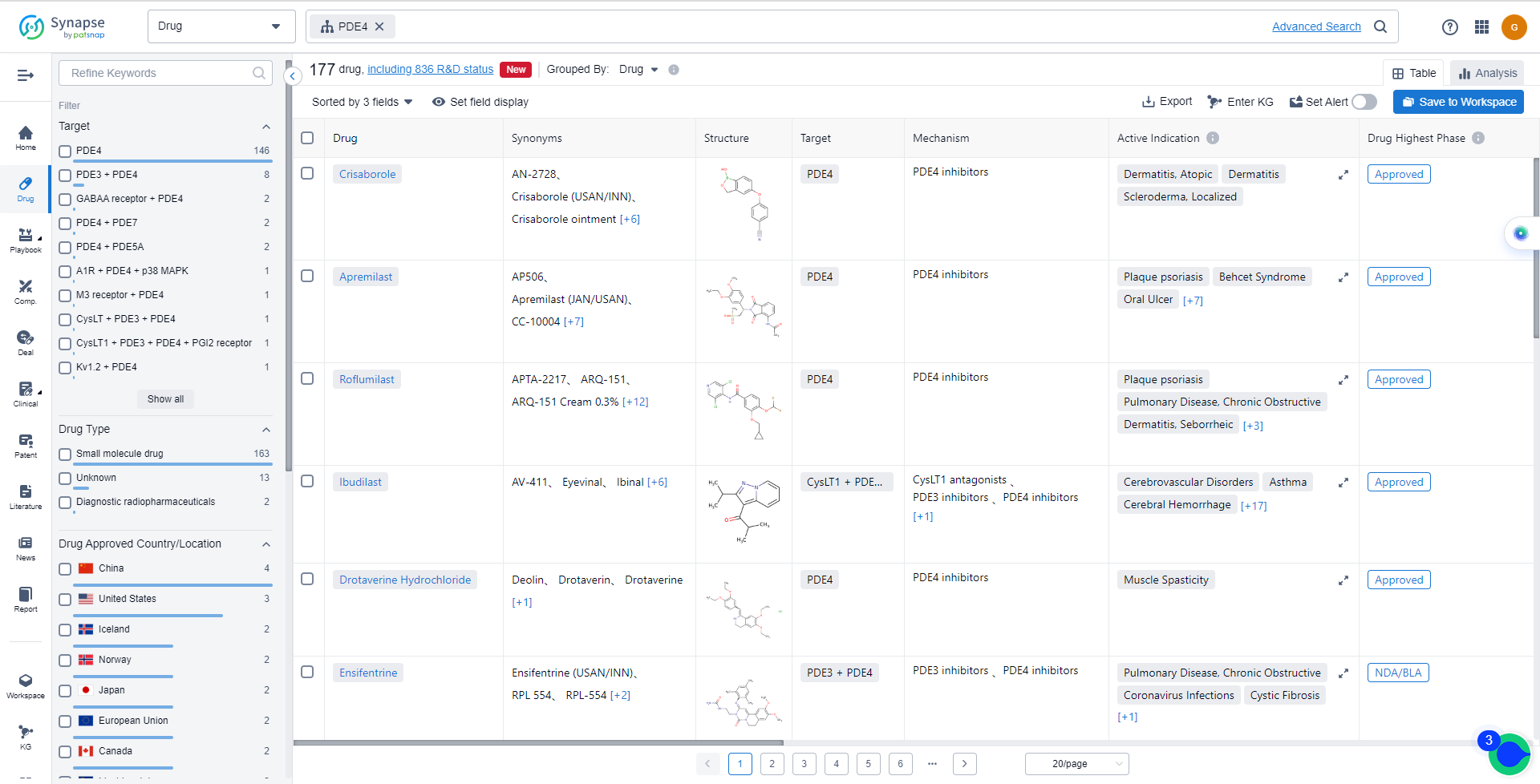
List of PDE4 Inhibitors
The currently marketed PDE4 inhibitors include:
- Crisaborole
- Apremilast
- Roflumilast
- Ibudilast
- Drotaverine Hydrochloride
- Ensifentrine
- HPP-737
- Mufemilast
- Tanimilast
- Zatolmilast
For more information, please click on the image below.
What are PDE4 inhibitors used for?
Clinical research on PDE4 inhibitors spans a variety of disease areas, including respiratory diseases, autoimmune diseases, central nervous system diseases, and skin diseases. For more information, please click on the image below to log in and search.
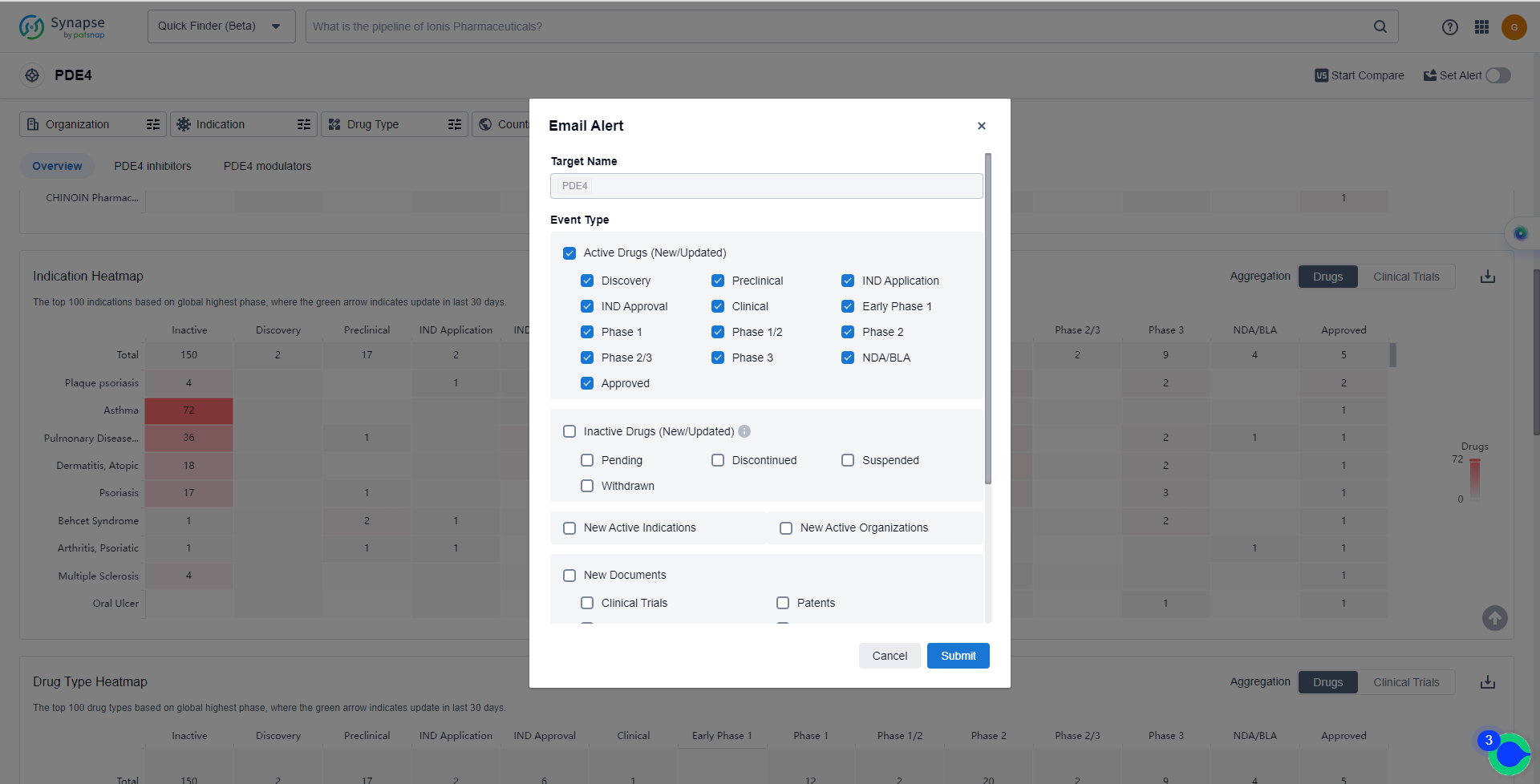
How to obtain the latest development progress of PDE4 inhibitors?
In the Synapse database, you can keep abreast of the latest research and development advances of PDE4 inhibitors anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!