Exploring Selinexor's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Selinexor's R&D Progress
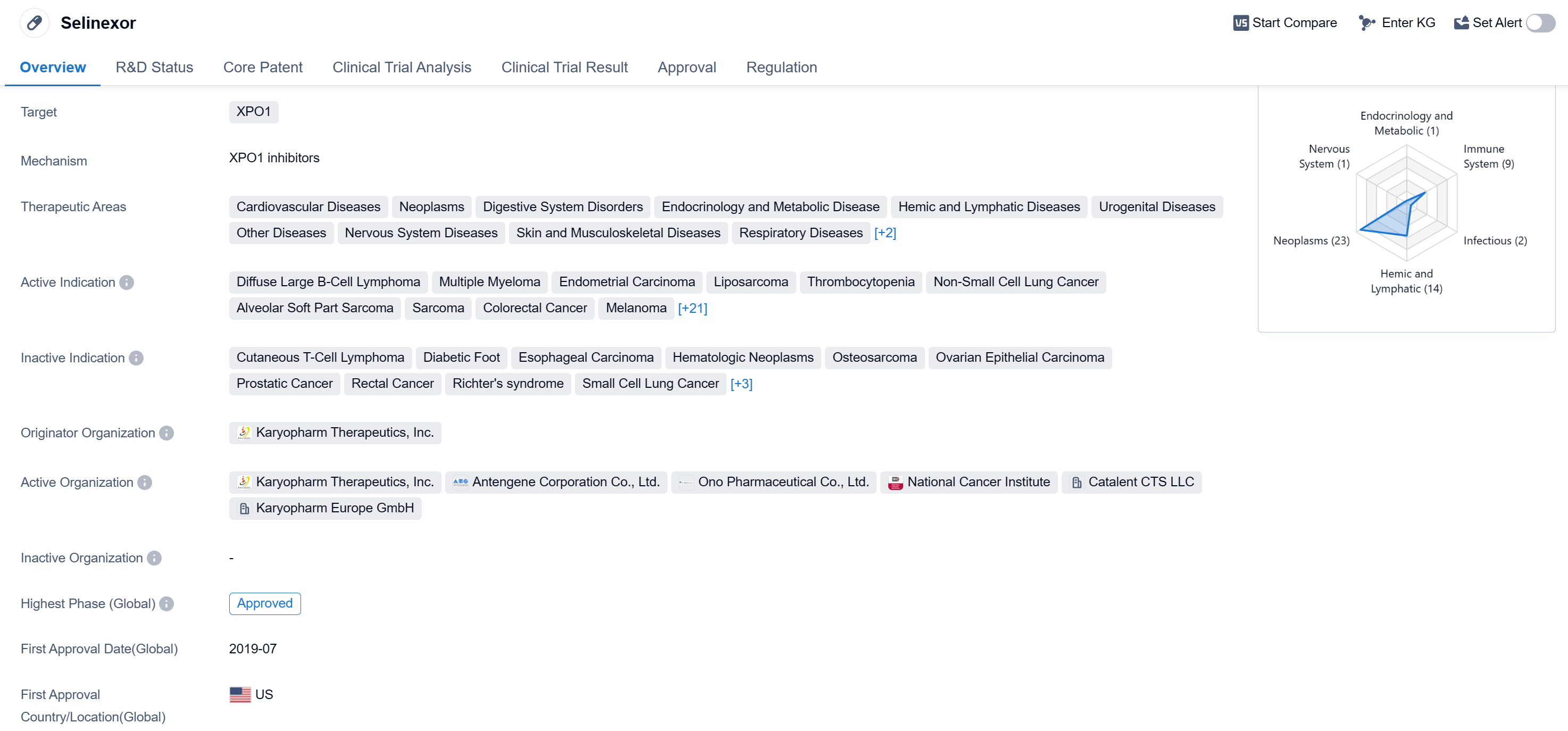
Selinexor is a small molecule drug developed by Karyopharm Therapeutics, Inc. It is classified as a targeted therapy that specifically targets the XPO1 protein. XPO1 plays a crucial role in the transport of certain proteins out of the nucleus, and its dysregulation has been implicated in various diseases.
The therapeutic areas in which Selinexor has shown potential include cardiovascular diseases, neoplasms (including various types of cancers), digestive system disorders, endocrinology and metabolic diseases, hemic and lymphatic diseases, urogenital diseases, nervous system diseases, skin and musculoskeletal diseases, respiratory diseases, immune system diseases, infectious diseases, and other diseases.
Selinexor has been approved for multiple indications, including diffuse large B-cell lymphoma, multiple myeloma, endometrial carcinoma, liposarcoma, thrombocytopenia, non-small cell lung cancer, alveolar soft part sarcoma, sarcoma, colorectal cancer, melanoma, osteomyelofibrosis, COVID-19, post-essential thrombocythemia myelofibrosis, post-polycythemia vera myelofibrosis, primary myelofibrosis, thymoma, thymic epithelial tumor, peripheral T-cell lymphoma, myelodysplastic syndromes, breast cancer, ovarian cancer, glioblastoma, acute myeloid leukemia, gastrointestinal stromal tumors, extranodal NK-T-cell lymphoma, non-Hodgkin lymphoma, mantle-cell lymphoma, prolymphocytic leukemia, small lymphocytic lymphoma, and B-cell chronic lymphocytic leukemia. It has also shown potential in the treatment of sepsis.
Selinexor received its first approval in the United States in July 2019 and has since been approved in other countries as well. It has been granted various regulatory designations, including orphan drug status, fast track designation, accelerated approval, and priority review. These designations highlight the potential of Selinexor to address unmet medical needs and expedite its development and availability to patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Selinexor: XPO1 inhibitors
XPO1 inhibitors are a type of drugs that target and inhibit the activity of the protein Exportin 1 (XPO1). XPO1 is responsible for transporting certain proteins out of the cell nucleus and into the cytoplasm. By inhibiting XPO1, these inhibitors prevent the export of specific proteins that play a role in cancer cell growth and survival.
From a biomedical perspective, XPO1 inhibitors are of great interest in the field of cancer research and treatment. They have shown potential as targeted therapies for various types of cancer, including solid tumors and hematological malignancies. By blocking the export of certain proteins, XPO1 inhibitors can disrupt cancer cell proliferation, induce cell death, and potentially enhance the effectiveness of other anticancer treatments.
XPO1 inhibitors have been studied in preclinical and clinical trials, and some have received regulatory approval for the treatment of specific cancers. Examples of XPO1 inhibitors include selinexor and verdinexor. These drugs are typically administered orally and are being investigated as both monotherapies and combination therapies in cancer patients.
Overall, XPO1 inhibitors hold promise as a new class of targeted therapies for cancer treatment by specifically interfering with the transport of proteins involved in cancer cell growth and survival.
Drug Target R&D Trends for Selinexor
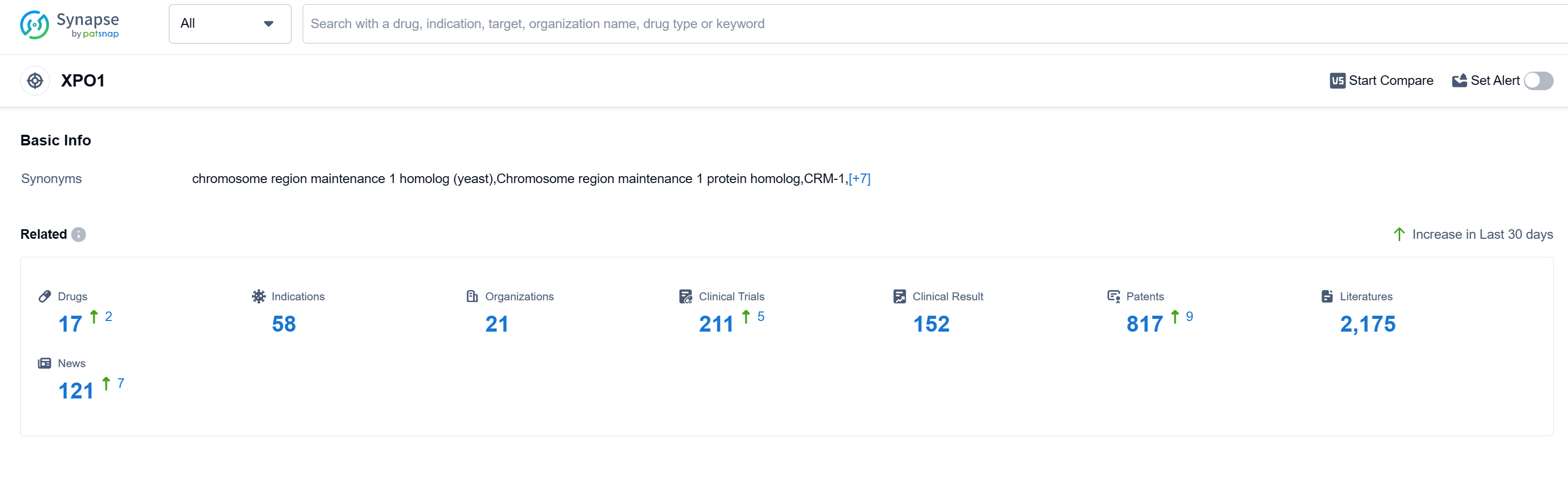
According to Patsnap Synapse, as of 15 Sep 2023, there are a total of 17 XPO1 drugs worldwide, from 21 organizations, covering 58 indications, and conducting 211 clinical trials.
The analysis of target XPO1 reveals a competitive landscape with multiple companies actively developing drugs under this target. Karyopharm Therapeutics, Inc., Antengene Corporation Co., Ltd., and Catalent, Inc. are among the companies growing fastest in this field. The R&D progress of these companies is evident from their diverse pipeline and drugs in different stages of development.
Several drugs under target XPO1 have been approved for indications such as Multiple Myeloma, Diffuse Large B-Cell Lymphoma, and Endometrial Carcinoma. This indicates the potential therapeutic value of XPO1-targeted therapies in these indications.
Small molecule drugs and chemical drugs are progressing rapidly under the target XPO1, with biosimilars also showing significant development. This suggests intense competition and ongoing research in the field.
The United States, China, and the European Union are the countries/locations developing fastest under the target XPO1. China, in particular, has shown progress in the development of XPO1-targeted therapies.
Overall, the analysis of target XPO1 provides insights into the current competitive landscape and future development prospects. The findings can guide pharmaceutical companies and researchers in their strategic decision-making and resource allocation for XPO1-targeted therapies.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Selinexor is a small molecule drug developed by Karyopharm Therapeutics, Inc. that targets the XPO1 protein. It has shown promise in treating a wide range of diseases, including various types of cancers, cardiovascular diseases, and infectious diseases. With its multiple approvals and regulatory designations, Selinexor represents a significant advancement in the field of biomedicine and offers hope for patients in need of effective treatments.