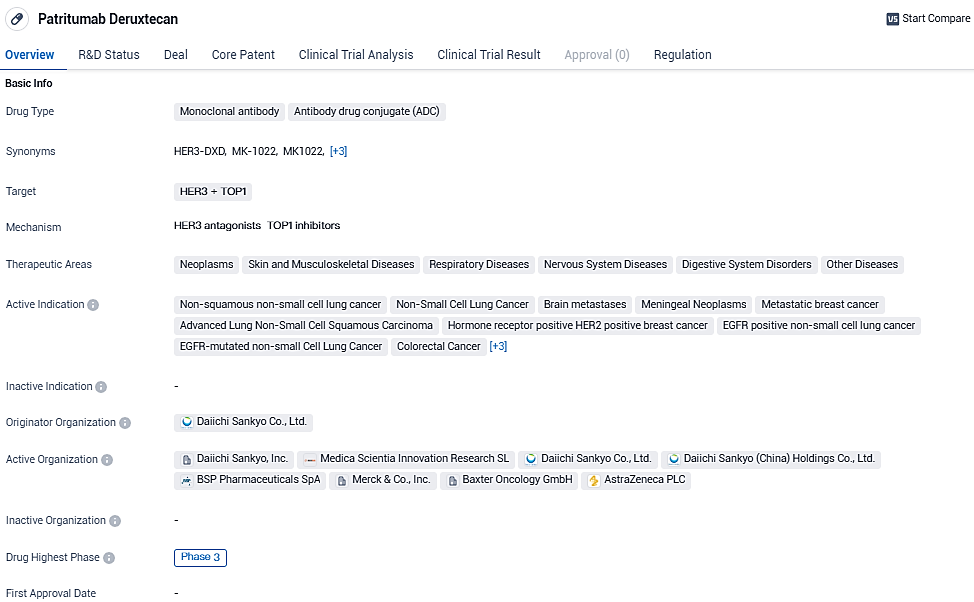
US health authorities fast-track review of Patritumab Deruxtecan for certain advanced EGFR-mutant Non-Small Cell Lung Cancer post-initial therapy
Daiichi Sankyo and Merck have declared that the Biologics License Application for patritumab deruxtecan (HER3-DXd) has garnered Priority Review status from the U.S. Food and Drug Administration. This application pertains to the use of this therapeutic agent in the care of adults who are dealing with either locally advanced or metastatic non-small cell lung cancer marked by EGFR mutations, and who have already undergone at least two systemic treatment regimens.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The target action date set by the FDA under the Prescription Drug User Fee Act for their assessment decision is scheduled for the 26th of June, 2024. Following the designation of Breakthrough Therapy received from the FDA in December 2021, the application now has the benefit of a Priority Review status.
The FDA prioritizes the review of drug applications that could potentially represent substantial advancements in treatment compared to existing therapies, either through showing an improvement in safety or effectiveness, averting severe health issues, or contributing to better adherence to treatments by patients.
The Biologics License Application (BLA) for patritumab deruxtecan is undergoing examination as part of the FDA’s Real-Time Oncology Review (RTOR) program, which aims to expedite the process of delivering new and effective anti-cancer drugs to patients in need. The RTOR framework permits the FDA to begin evaluating certain sections of a drug’s application ahead of the full package being submitted.
Patritumab deruxtecan, which is being co-developed and marketed by Daiichi Sankyo in partnership with Merck, is an innovative potential leading agent targeting HER3 with a DXd antibody-drug conjugate format. This therapy was originally identified by Daiichi Sankyo.
Data underpinning the BLA include findings from the central assessment of the HERTHENA-Lung01 Phase 2 study, alongside outcomes showcased at the IASLC World Conference on Lung Cancer in 2023. These findings were also concurrently released in the Journal of Clinical Oncology.
Commenting on the FDA’s prioritized handling of the BLA, Ken Takeshita, MD, who is the Global Head of R&D at Daiichi Sankyo, mentioned, “The expedited review by the FDA underscores the compelling nature of the data emerging from the HERTHENA-Lung01 study, and it highlights the urgency in delivering novel therapeutic alternatives to individuals battling advanced stages or metastatic instances of EGFR-mutated non-small cell lung cancer after undergoing two or more systemic treatment rounds.”
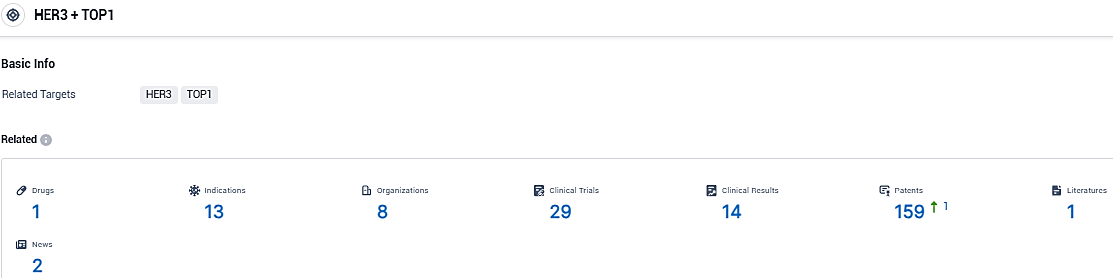
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 30, 2023, there are 1 investigational drugs for the HER3 and TOP1 target, including 13 indications, 8 R&D institutions involved, with related clinical trials reaching 29, and as many as 159 patents.
Patritumab deruxtecan was granted Breakthrough Therapy Designation by the U.S. Food and Drug Administration in December 2021 for the treatment of patients with EGFR-mutated locally advanced or metastatic NSCLC with disease progression on or after treatment with a third-generation TKI and platinum-based therapies.