Constant Therapeutics Reveals Initial Participant Receives Treatment in Stage 2 Study for Stroke Rehab using Drug TXA127
Constant Therapeutics LLC, a company specializing in the biopharmaceutical field dedicated to formulating therapies that target the Alternative Renin-Angiotensin System, has recently revealed that the inaugural patient has received the initial dose in the firm's phase 2 clinical study. This trial investigates the efficacy of TXA127, the principal peptide-based therapeutic candidate the company is advancing, which is designed to potentially enhance the recuperation process following an ischemic stroke.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The second-phase clinical trial utilizes a methodical approach, incorporating random selection, the use of placebos, a double-masked procedure, and leveraging a semi-decentralized format. Its purpose is to ascertain the level of safety and effectiveness of treatment in individuals who have experienced an ischemic stroke, targeting an age demographic ranging from 18 to 85 years old, and being within a 6- to 24-month timeframe following their stroke event.
Throughout a 90-day period, participants will undergo therapeutic intervention. The research is taking place within the Sheba Medical Center in Israel, where it operates in partnership with Sabar Health for administering in-home patient care. The goal is to include a total of 50 individuals in this study.
Rick Franklin, the Chief Executive Officer of Constant Therapeutics, has stated, "The arena of persistent stroke treatment currently lacks pharmacological solutions, and conducting clinical studies for this condition presents a significant challenge. We consider ourselves privileged to establish the requisite foundation, alongside Sabar Health and Sheba Medical Center for the infrastructure. This distinct collaboration equips us to carry out trials on TXA127, aiming to address this critical healthcare gap."
TXA127 constitutes a medicinal blend modeled after the human peptide angiotensin-(1-7), a compound occurring naturally in the body. TXA127 not only has potential applications in ischemic stroke but has also displayed positive results in preclinical studies for a range of muscular and connective tissue disorders, including Duchenne Muscular Dystrophy, Limb-Girdle Muscular Dystrophy, Congenital Muscular Dystrophy, Marfan Syndrome, and Epidermolysis Bullosa.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
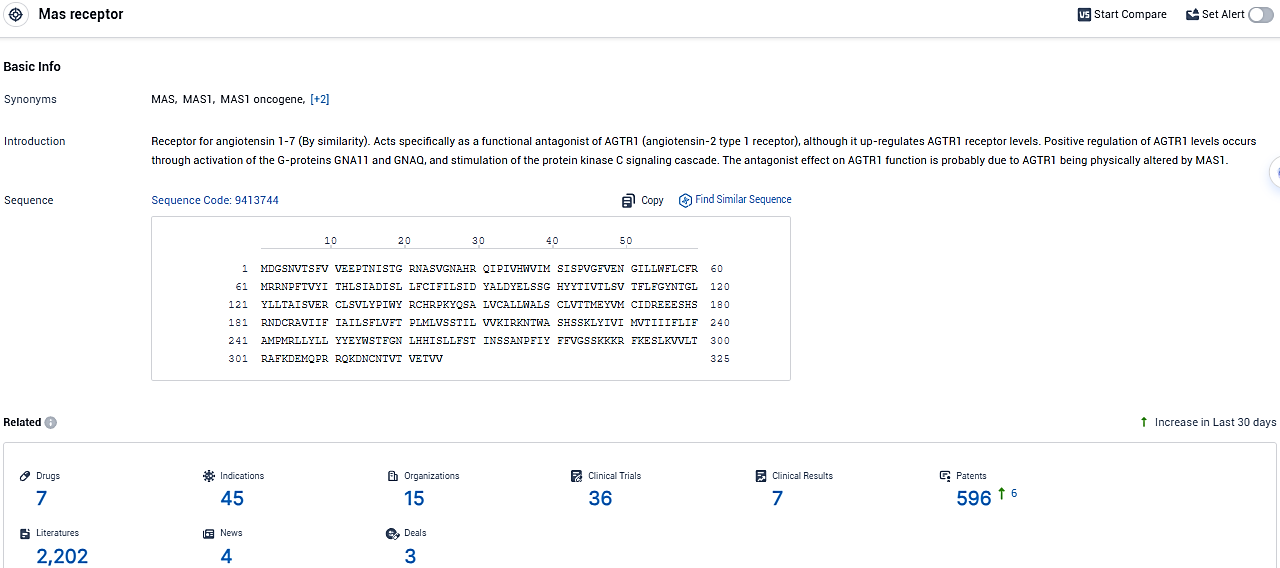
According to the data provided by the Synapse Database, As of January 15, 2024, there are 7 investigational drugs for the Mas receptor, including 45 indications, 15 R&D institutions involved, with related clinical trials reaching 36, and as many as 596 patents.
TXA127 is a synthetic peptide drug targeting the Mas receptor, currently in Phase 2 of clinical development. The drug's active indications cover conditions such as ischemic stroke, muscular dystrophy, epidermolysis bullosa, amyotrophic lateral sclerosis, and Marfan syndrome. TXA127 represents a promising option in the field of biomedicine, particularly for orphan diseases.