Vericel Announces FDA Approval and Market Release of MACI Arthro
Vericel Corporation (NASDAQ:VCEL), a key player in the field of advanced therapies for sports medicine and critical burn care, has announced that the U.S. Food and Drug Administration (FDA) has accepted a supplemental Biologics License Application (sBLA). This approval broadens the scope of the MACI® (autologous cultured chondrocytes on porcine collagen membrane) label, now permitting the use of arthroscopic techniques to deliver MACI for the treatment of symptomatic single or multiple full-thickness cartilage defects in the knee, with a maximum size of up to 4 cm². The new MACI Arthro™ method offers a less invasive option compared to existing procedures, enabling surgeons to inspect and prepare the defect area and administer the MACI implant through small incisions utilizing specially designed MACI Arthro instruments.
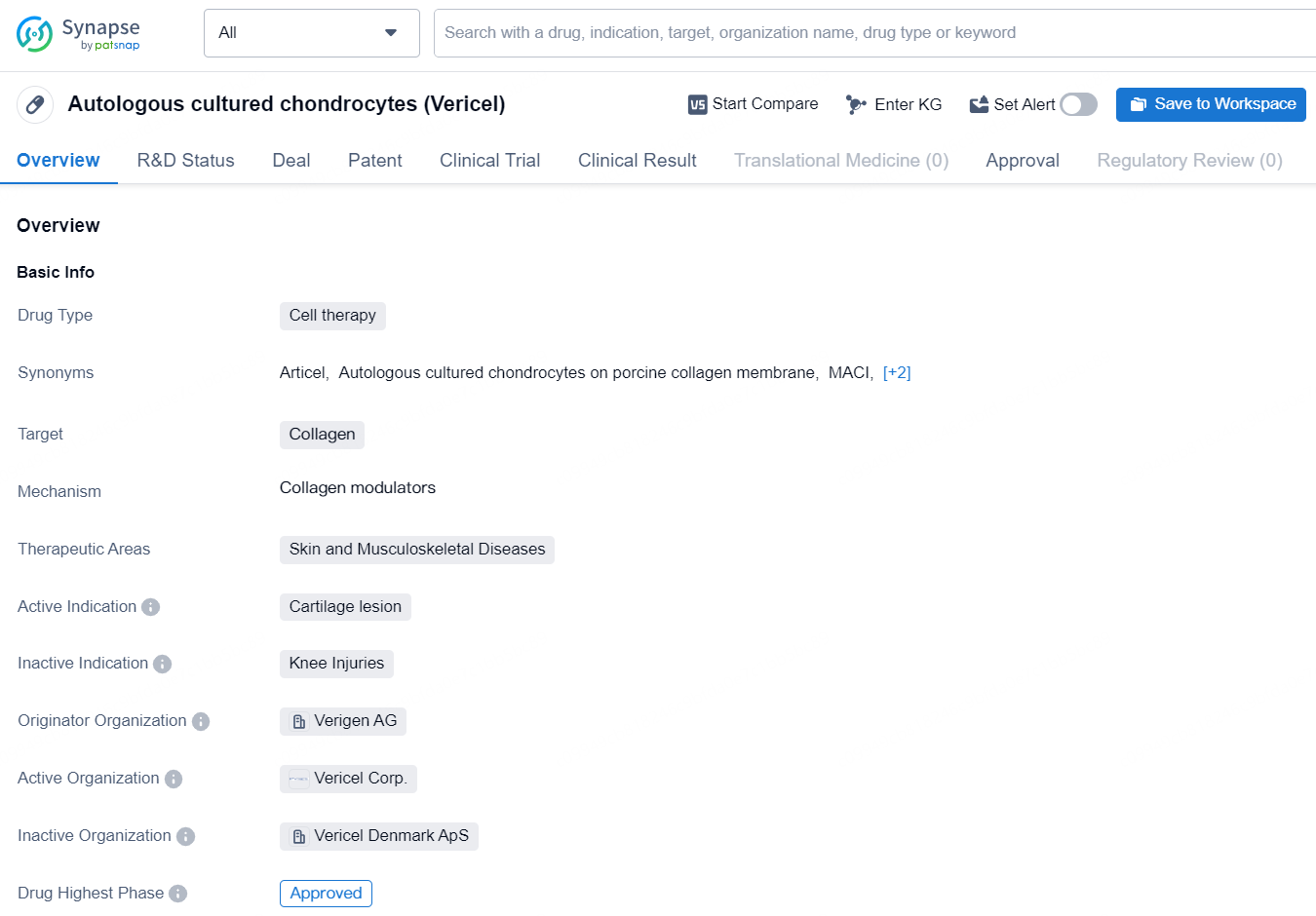
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The introduction of MACI Arthro marks a critical step forward in our mission to deliver novel treatments for patients experiencing pain and dysfunction due to knee cartilage defects," commented Nick Colangelo, President and CEO of Vericel. "MACI Arthro offers orthopedic surgeons and their patients a minimally invasive option for MACI delivery, which we believe could significantly enhance our entry into the largest segment of the MACI target market, thereby supporting ongoing top-tier revenue growth for the Company in the foreseeable future."
MACI is the first FDA-approved cellularized scaffold product utilizing tissue engineering methods to cultivate cells on scaffolds using healthy cartilage tissue from the patient's own knee. It stands as the exclusive biologic cartilage repair solution approved for arthroscopic use. MACI Arthro combines the benefits of an arthroscopic approach with the proven durability and clinical efficacy of MACI.
The specialized MACI Arthro tools are crafted to address the most prevalent defects in the MACI target market, specifically 2-4cm2 defects on the femoral condyles, which affect around 20,000 patients annually or one-third of the $3 billion MACI market. Accompanying the rollout of MACI Arthro, Vericel is broadening its target base of surgeons from 5,000 to 7,000, including those who perform a high volume of cartilage repair surgeries, mainly through arthroscopy.
"Arthroscopic delivery of MACI is a major advancement in the field of cartilage repair," stated Grant H. Garcia, MD, of Orthopedic Specialists of Seattle. "This technique, along with the specially designed MACI Arthro instruments, offers surgeons a less invasive option to provide a clinically-proven treatment to patients, potentially being more favorable for patients due to the numerous post-operative benefits of arthroscopic surgery compared to open surgery."
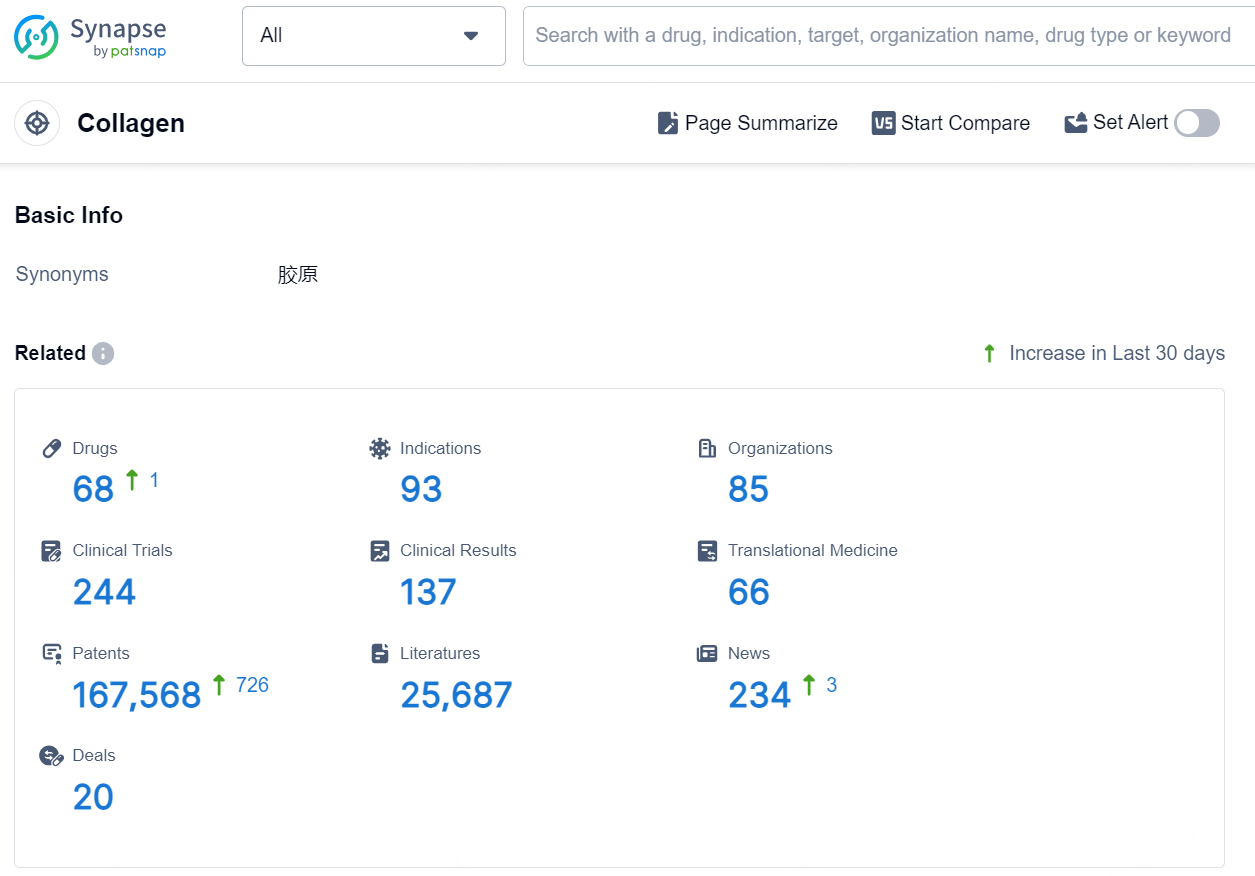
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 29, 2024, there are 68 investigational drugs for the Collagen targets, including 93 indications, 85 R&D institutions involved, with related clinical trials reaching 244, and as many as 167568 patents.
Autologous cultured chondrocytes, marketed under the trade name Vericel, is a cell therapy drug developed by Verigen AG. The drug is primarily targeted at collagen and is used to treat cartilage lesions, making it particularly effective in the therapeutic areas of skin and musculoskeletal diseases.