Verrica Pharmaceuticals Presents Positive Early Data from Phase 2 of VP-315 Basal Cell Carcinoma Treatment Study
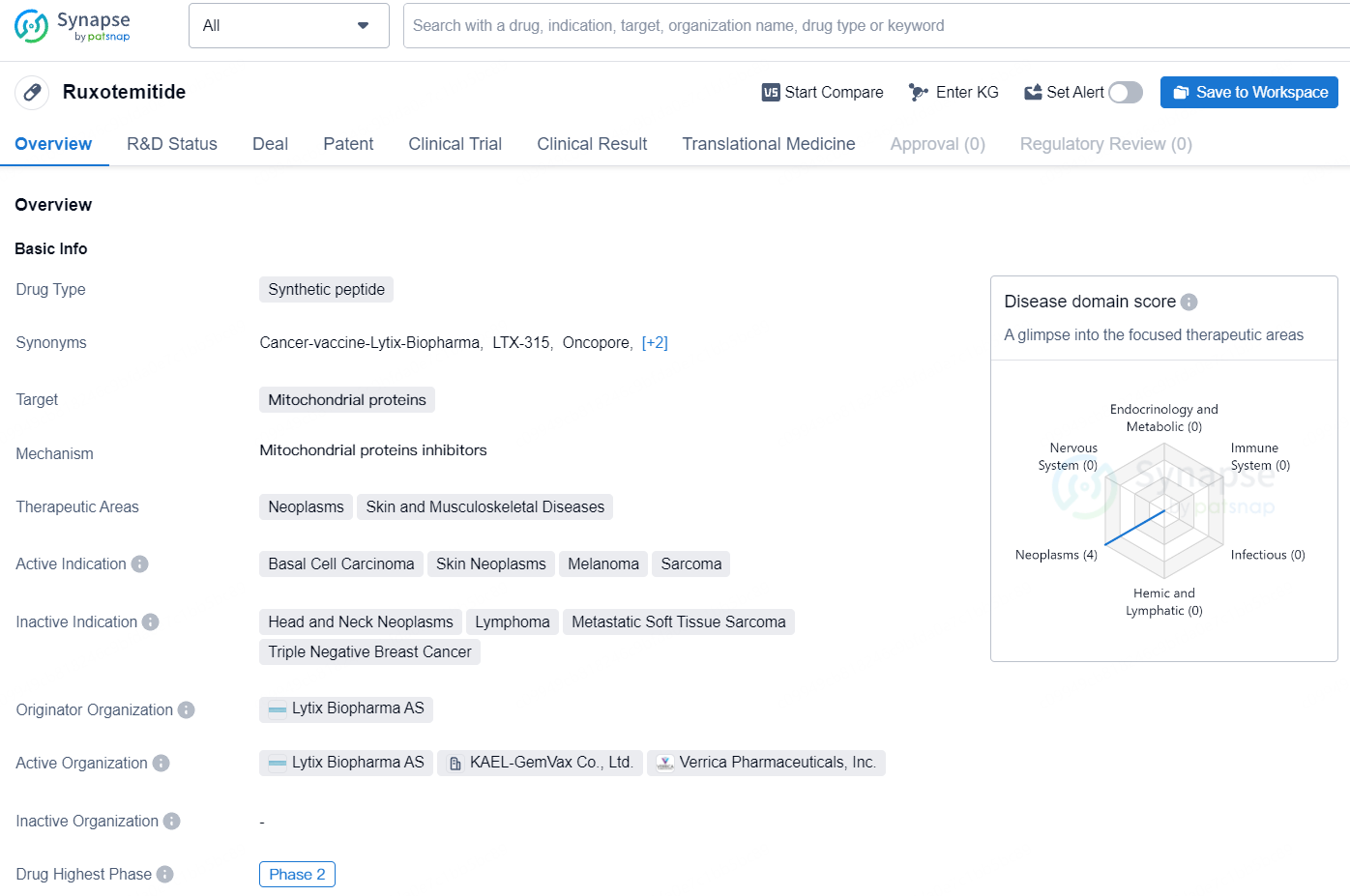
Verrica Pharmaceuticals Inc. ("Verrica" or "the Company") (Nasdaq: VRCA), a firm specializing in dermatology therapeutics, has revealed encouraging initial outcomes from Part 2 of its Phase 2 clinical study on VP-315(Ruxotemitide). This investigational oncolytic peptide, regarded as potentially groundbreaking, aims to treat basal cell carcinoma.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 ”We view the positive outcomes from Part 2 of the Phase 2 study for VP-315 as a significant advancement in potentially offering new treatment options for basal cell carcinoma patients,” said Ted White, President and CEO of Verrica. “The preliminary results are promising and suggest that VP-315 could be used as a first-line therapy in both primary and neoadjuvant settings. We believe VP-315 represents a substantial commercial opportunity, potentially worth billions for Verrica.”
”We view the positive outcomes from Part 2 of the Phase 2 study for VP-315 as a significant advancement in potentially offering new treatment options for basal cell carcinoma patients,” said Ted White, President and CEO of Verrica. “The preliminary results are promising and suggest that VP-315 could be used as a first-line therapy in both primary and neoadjuvant settings. We believe VP-315 represents a substantial commercial opportunity, potentially worth billions for Verrica.”
“Basal cell carcinoma is the most prevalent type of cancer in the United States, and current treatments have limitations, such as systemic side-effects,” commented Dr. Gary Goldenberg, Verrica’s Chief Medical Officer. “The early results from Part 2 of the trial indicate over 50% complete histological clearance and a notable decrease in tumor size in treated lesions, a result that may significantly enhance patient outcomes compared to existing therapies and surgical interventions. Additionally, due to the immunomodulatory properties of VP-315, we are eager to further investigate these attributes in tissue and blood samples.”
“VP-315 could potentially revolutionize the treatment of basal cell skin cancer by dermatologists. Patients might be cured with simple VP-315 injections,” stated Dr. Jonathan Kantor, dermatologist and Mohs surgeon at Florida Center for Dermatology, the principal enrollment site for the clinical trial. “Patients who still have residual tumor can undergo surgery with a reduction in surgical scar size by more than 70%, which is exceptionally promising.”
The Phase 2 trial is a multi-center, open-label, dose-escalation, proof-of-concept study with a safety run-in phase, aimed at evaluating the safety, tolerability, pharmacokinetics, and efficacy of intratumorally administered VP-315 in adults with biopsy-confirmed basal cell carcinoma. The study enrolled 92 adults with a histological diagnosis of basal cell carcinoma in at least one targeted lesion. More details about this clinical trial can be found at clinicaltrials.gov, under identifier NCT05188729.
Preliminary results from Part 2 are based on 93 confirmed basal cell carcinoma lesions treated in this part of the Phase 2 trial; however, data for histological reduction and overall reduction in tumor size are still pending for three of the 93 lesions. The key preliminary results from Part 2 include:
No dose-limiting toxicities or Treatment-Related Serious Adverse Events were reported. Most Treatment-Related Adverse Events were mild to moderate cutaneous reactions (n=93).
51% of treated lesions achieved complete histological clearance with no remaining tumor cells (n=93).
Histological reduction in tumor size for subjects with residual tumor was 71% (n=90).
All patients treated with VP-315 experienced a reduction in tumor size, with an overall reduction in tumor size across all subjects (both with residual and no residual tumor) of 86% (n=90).
The Company anticipates genomic and T-cell (immune response) data in Q1 2025 and intends to request an End-of-Phase 2 meeting with the FDA to discuss the next steps for VP-315's development for BCC in H1 2025. Additionally, the Company plans to present final Phase 2 data at upcoming medical conferences.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
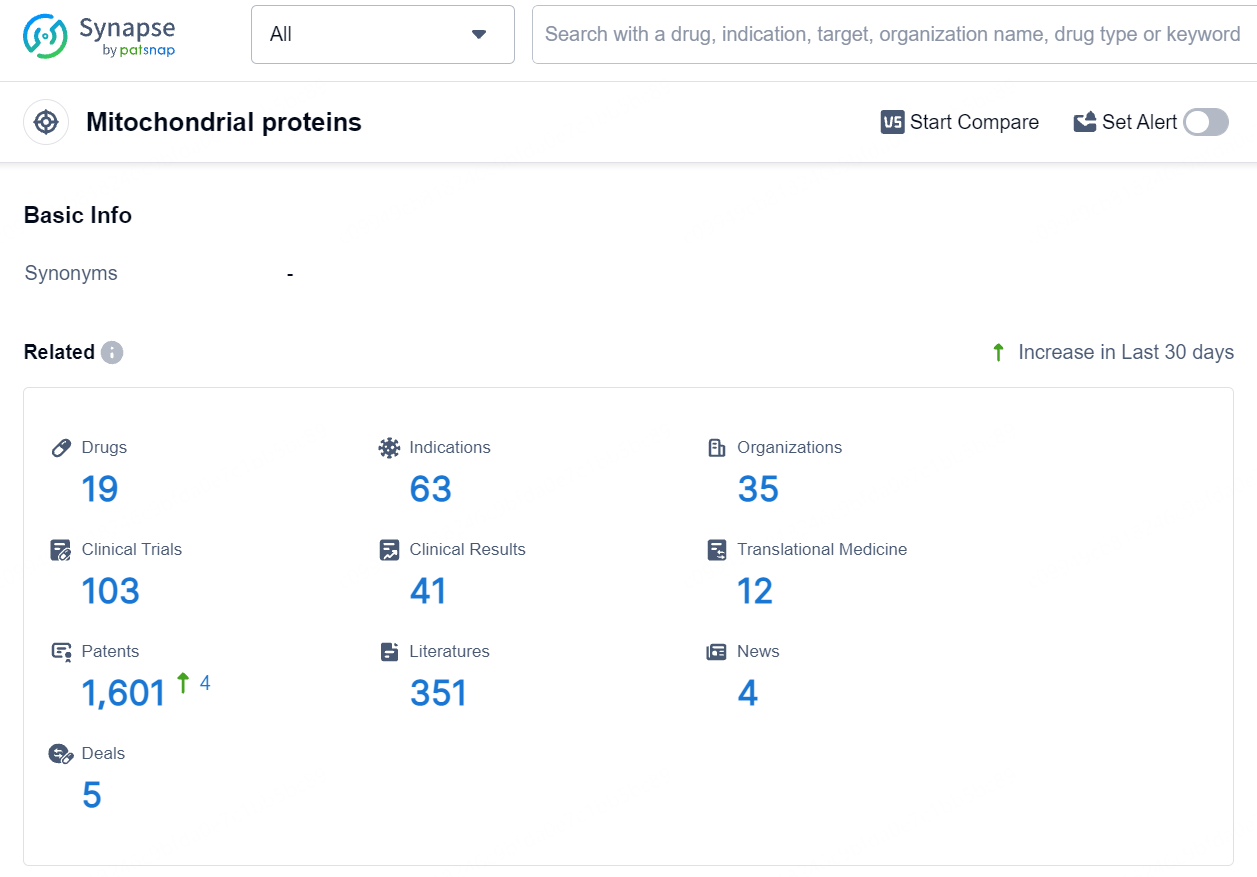
According to the data provided by the Synapse Database, As of August 15, 2024, there are 19 investigational drugs for the Mitochondrial proteins target, including 63 indications, 35 R&D institutions involved, with related clinical trials reaching 103, and as many as 1601 patents.
VP-315 represents a potential groundbreaking oncolytic peptide immunotherapy designed for direct intratumoral injection, aimed at prompting immunogenic cell death. This could provide a non-surgical treatment alternative for skin cancer patients. Rooted in advanced research on “host defense peptides,” which constitute nature's primary defense against foreign pathogens, VP-315 is introduced directly into the tumor as a chemotherapeutic agent. It induces the breakdown of intracellular organelles in tumor cells, such as the mitochondria, thereby releasing a variety of tumor antigens to provoke T cell responses. Verrica holds an exclusive global license to develop and market VP-315 for dermatologic oncology, targeting conditions such as non-metastatic melanoma and non-metastatic merkel cell carcinoma. The company plans to initially concentrate on basal cell and squamous cell carcinomas as priority indications for further development.