Bavarian Nordic's Chikungunya Vaccine BLA Accepted and Granted Priority Review by FDA
Bavarian Nordic A/S (OMX: BAVA) announced today that the U.S. Food and Drug Administration (FDA) has accepted and given Priority Review status to the Biologics License Application (BLA) for CHIKV VLP(PXVX-0317), the Company's vaccine candidate designed to immunize individuals aged 12 and above against disease caused by chikungunya virus infection.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The FDA's Priority Review designation aims to finalize its evaluation within six months compared to the standard review period of 10 months, setting a Prescription Drug User Fee Act (PDUFA) action date for February 14, 2025.
"We are pleased with the FDA’s decision to grant Priority Review for our chikungunya vaccine, which allows for a more expedited review process. We anticipate collaborating closely with regulators to ensure our vaccine is accessible to individuals aged 12 and older who are at risk of chikungunya virus infection. The FDA review, along with the ongoing EMA evaluation of our CHIKV VLP vaccine, marks the initial regulatory assessments of a chikungunya vaccine for adolescents, potentially enabling broader use among at-risk populations," commented Paul Chaplin, President and CEO of Bavarian Nordic.
In addition, the CHIKV VLP vaccine is under accelerated assessment review by the European Medicines Agency (EMA), which could lead to European Commission approval in the first half of 2025.
About the CHIKV VLP Vaccine
CHIKV VLP is an adjuvanted VLP-based vaccine candidate aimed at active immunization to prevent diseases caused by CHIKV infection. Subject to regulatory approval, the single-dose vaccine will be available in a pre-filled syringe to facilitate easier administration, thus saving time for vaccinators and minimizing the risk of administrative mistakes.
The CHIKV VLP vaccine candidate has previously received Breakthrough Therapy designation and Fast Track designation from the FDA in October 2020 and April 2018, respectively, as well as PRIME designation from the EMA in September 2019. These designations aim to facilitate the development or expedite the review of medicines that either address an unmet medical need or could provide significant improvement over existing treatments. In February 2024, the Committee for Medicinal Products for Human Use (CHMP) under EMA granted accelerated assessment for the MAA for the CHIKV VLP vaccine candidate, recognizing its significant public health interest and therapeutic innovation.
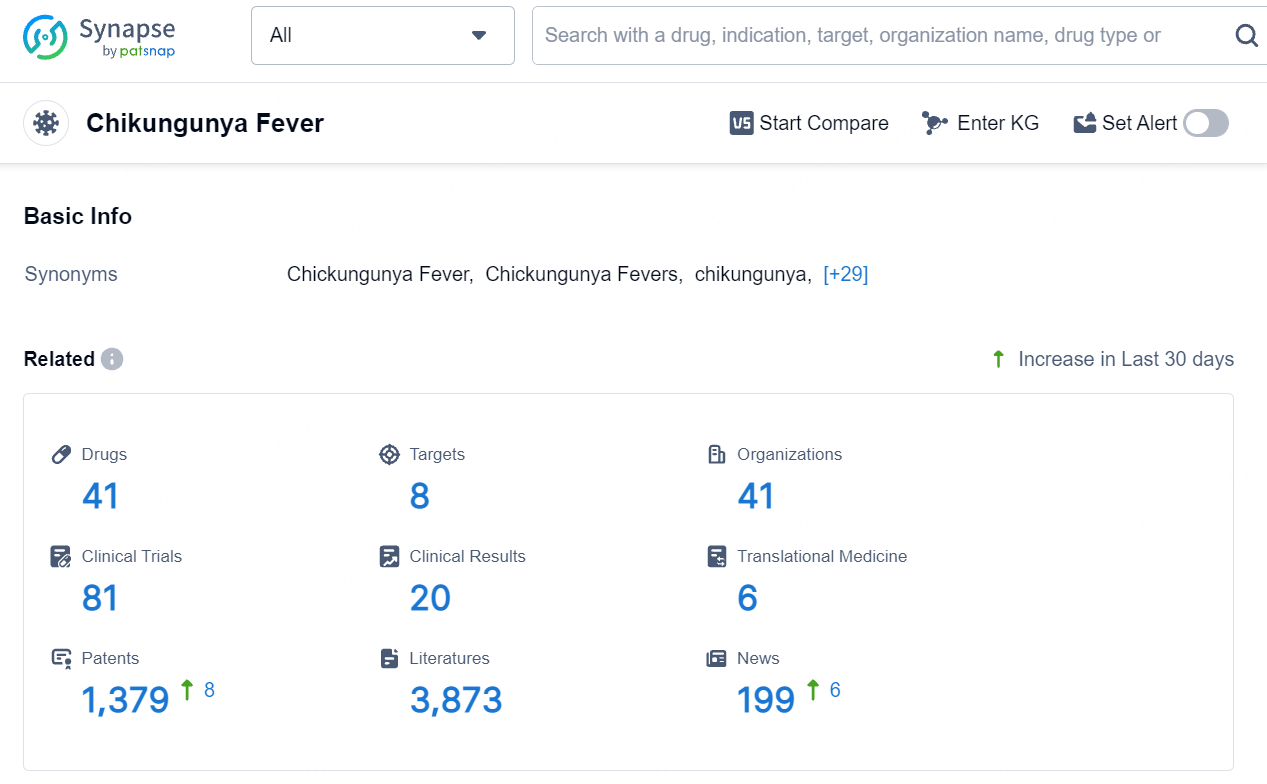
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 15, 2024, there are 41 investigational drugs for the Chikungunya Fever, including 8 targets, 41 R&D institutions involved, with related clinical trials reaching 81, and as many as 1379 patents.
Chikungunya is a mosquito-borne viral disease caused by the chikungunya virus (CHIKV), which belongs to the group of arboviruses like dengue virus. CHIKV disease typically presents with acute symptoms, including fever, rash, fatigue, headache, and often severe and incapacitating joint pain. While mortality is relatively low, morbidity is high; nearly 50% of individuals with CHIKV disease have debilitating long-term symptoms that can intensify with age. In the past 20 years, the CHIKV has emerged in several previously non-endemic regions in Asia, Africa, southern Europe, and the Americas, often causing large unpredictable outbreaks. Recent data1 suggest that chikungunya is severely underreported and often misdiagnosed as dengue fever due to lack of proper testing.