Weekly Insulin Efsitora Alfa Matches Daily Insulin in A1C Reduction for Type 1 Diabetes
Eli Lilly and Company (NYSE: LLY) has released comprehensive findings from the QWINT-5 phase 3 study, which compared the efficacy of once-weekly insulin efsitora alfa (efsitora) to once-daily insulin degludec in adults with type 1 diabetes who need daily basal plus multiple daily mealtime insulin injections. These results were published in The Lancet and presented at the European Association for the Study of Diabetes (EASD) Annual Meeting 2024.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
In the study, efsitora achieved the primary efficacy endpoint by demonstrating a comparable reduction in A1C levels at week 26. Regarding the efficacy estimand, efsitora lowered A1C by 0.53%, while insulin degludec achieved a 0.59% reduction, resulting in final A1C values of 7.37% and 7.32%, respectively.
As for a key secondary endpoint, the time in range as assessed by continuous glucose monitoring (CGM) was comparable between efsitora and insulin degludec in the four weeks leading up to week 26. Another significant secondary endpoint indicated that the estimated combined rates of patient-reported clinically significant or severe nocturnal hypoglycemic events (blood glucose <54 mg/dL) per patient-year of exposure were similar between efsitora and insulin degludec throughout the 52-week study duration.
"Individuals with type 1 diabetes require daily insulin. Currently, they can administer insulin via an automated insulin delivery system or by combining daily basal insulin injections with multiple mealtime insulin injections," said Richard Bergenstal, M.D., the executive director of the International Diabetes Center at HealthPartners Institute. "These new findings indicate that a once-weekly dose of basal insulin, efsitora, can provide A1C reductions comparable to daily injections of one of the most commonly used basal insulins. I am eager to see further analysis of this data, including strategies to reduce hypoglycemia episodes, to explore once-weekly insulin as a potential personalized treatment approach for type 1 diabetes."
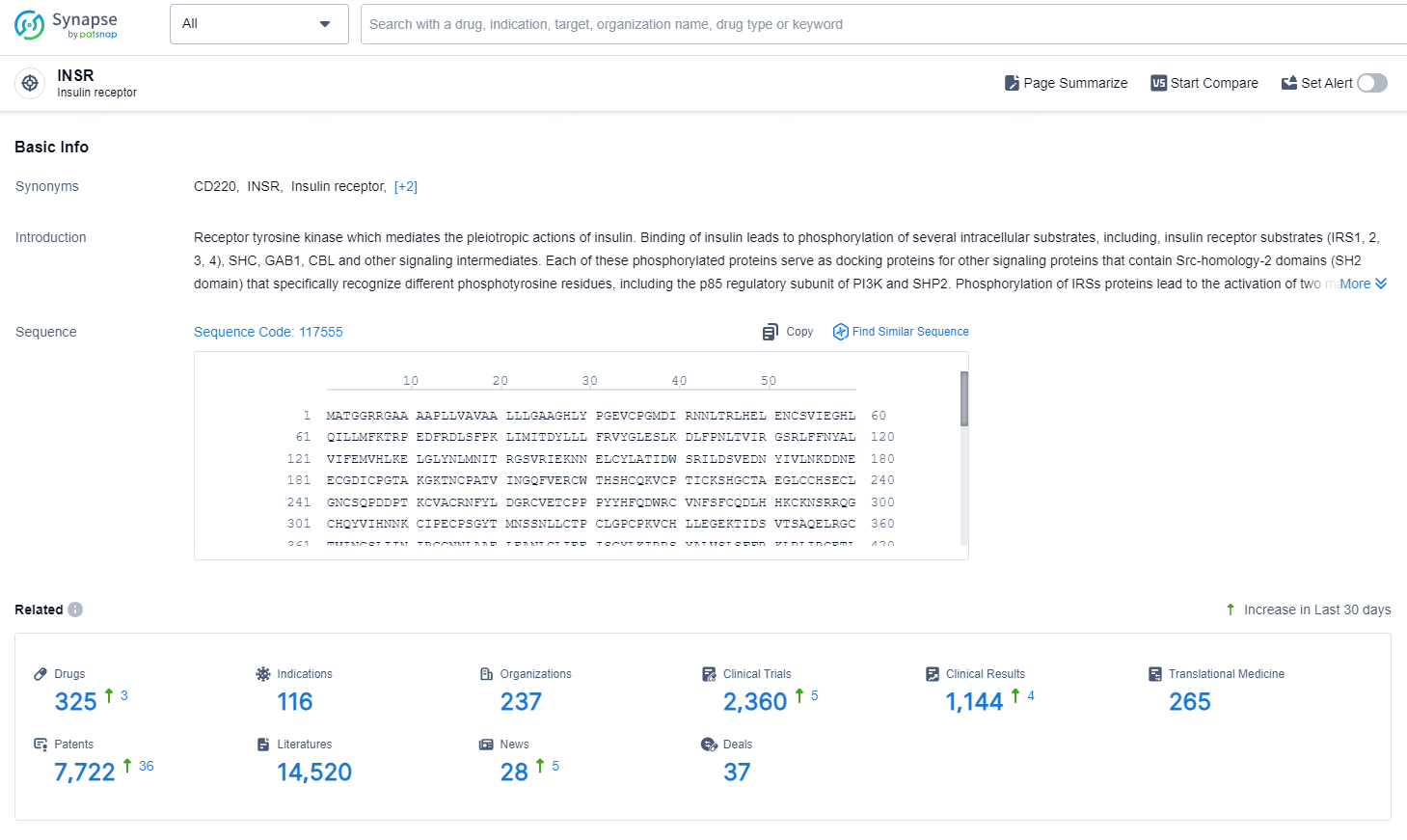
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 12, 2024, there are 325 investigational drugs for the INSR targets, including 116 indications, 237 R&D institutions involved, with related clinical trials reaching 2360, and as many as 7722 patents.
The drug Basal Insulin Fc, developed by Eli Lilly & Co., belongs to the categories of hormone and fusion protein. It targets the INSR (Insulin Receptor) and is intended for the treatment of diseases related to the immune system, as well as endocrinology and metabolic disorders. The active indications for this drug include Diabetes Mellitus Type 1, Diabetes Mellitus Type 2, and Hypoglycemia.