Xilio Therapeutics Starts Phase 1 Trial Enrollment for XTX101 Combo and Updates Monotherapy Data
Xilio Therapeutics, Inc. has launched the participant recruitment process for a Phase 1 trial, where XTX101—a cancer-activated, Fc-modified anti-CTLA-4 antibody—will be tested in combination with other treatments. Additionally, the company has released revised data from its Phase 1 study where XTX101 was used as a single-agent therapy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
XTX101 is currently under examination as a tumor-triggered, Fc-amplified monoclonal antibody targeting CTLA-4, and is being studied alongside atezolizumab. The most recent solo treatment findings come from the active Phase 1 study that is deployed to assess XTX101 in patients facing advanced-stage cancer characterized by resistance to immuno-oncology treatments.
Katarina Luptakova, M.D., and Chief Medical Officer at Xilio Therapeutics, stated, “Following the recent commencement of the Phase 1 ascending dose study of XTX101 combined with atezolizumab, we are eager to define an optimal Phase 2 dose. Our aim is to further explore this combination’s potential in a subsequent Phase 2 study focusing on individuals grappling with microsatellite stable colorectal cancer, a patient group facing particularly pressing medical needs, including those with liver metastasis.”
She continued, “The fresh Phase 1 data surrounding XTX101 reflects its favorable tolerability profile, with adverse reactions predominantly falling within Grade 1 or 2. In addition to a previously noted confirmed partial response enduring 36 weeks in a patient with non-small cell lung cancer, this new set of data gives credence to the anti-cancer activity of XTX101 as a standalone therapy. Particularly, this is evident in late-stage and immuno-oncology therapy-resistant patients, administered at the prospective Phase 2 treatment level.”
As a high-affinity anti-CTLA-4 therapeutic antibody, XTX101 is engineered to not only inhibit CTLA-4 but also deplete T regulatory cells specifically within the tumor’s microenvironment upon activation. XTX101's Phase 1 study is pioneering in its approach as a human trial, is multi-center in nature, and does not mask its open-label design. The main goals are to gauge the safety and tolerance of XTX101 in adults with advanced solid tumors. Presently, Xilio has achieved its targeted participant enrollment for the dose-escalation portion of the monotherapy assessment.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
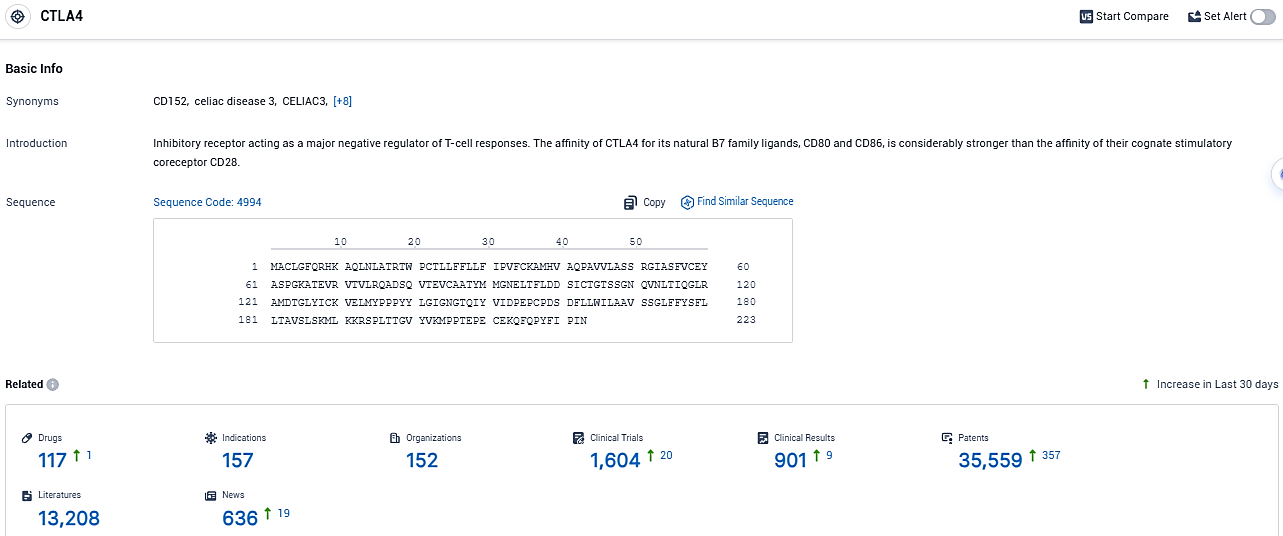
According to the data provided by the Synapse Database, As of December 15, 2023, there are 117 investigational drugs for the CTLA-4 target, including 157 indications, 152 R&D institutions involved, with related clinical trials reaching 1604, and as many as 35559 patents.
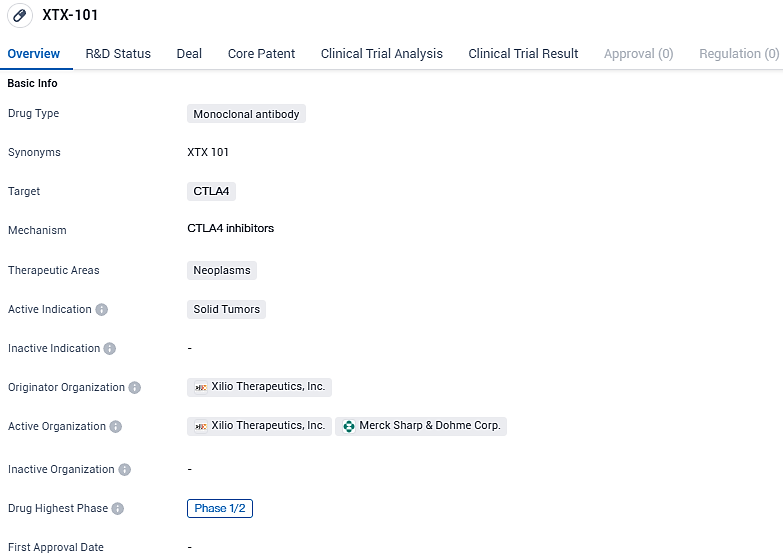
XTX-101 targets CTLA4 and is intended for the treatment of neoplasms, specifically solid tumors. As of the latest available information, XTX-101 is in Phase 1/2 of clinical development. Further research and clinical trials will be necessary to determine the potential of XTX-101 as a therapeutic option for patients with solid tumors.