XyloCor Therapeutics Announces Positive Phase 2 Results for XC001 at SCAI 2024, Published in Circulation: Cardiovascular Interventions
XyloCor Therapeutics, Inc., a biopharmaceutical firm in the clinical stage that is pioneering new gene therapy treatments for cardiovascular disease, announced definitive outcomes from the Phase 2 segment of its Phase 1/2 clinical study for its primary gene therapy candidate, XC001 (encoberminogene rezmadenovec), targeting refractory angina. This was revealed during the Scientific Sessions of the Society for Cardiovascular Angiography & Interventions, held from May 2 to 4, 2024 in Long Beach, CA.
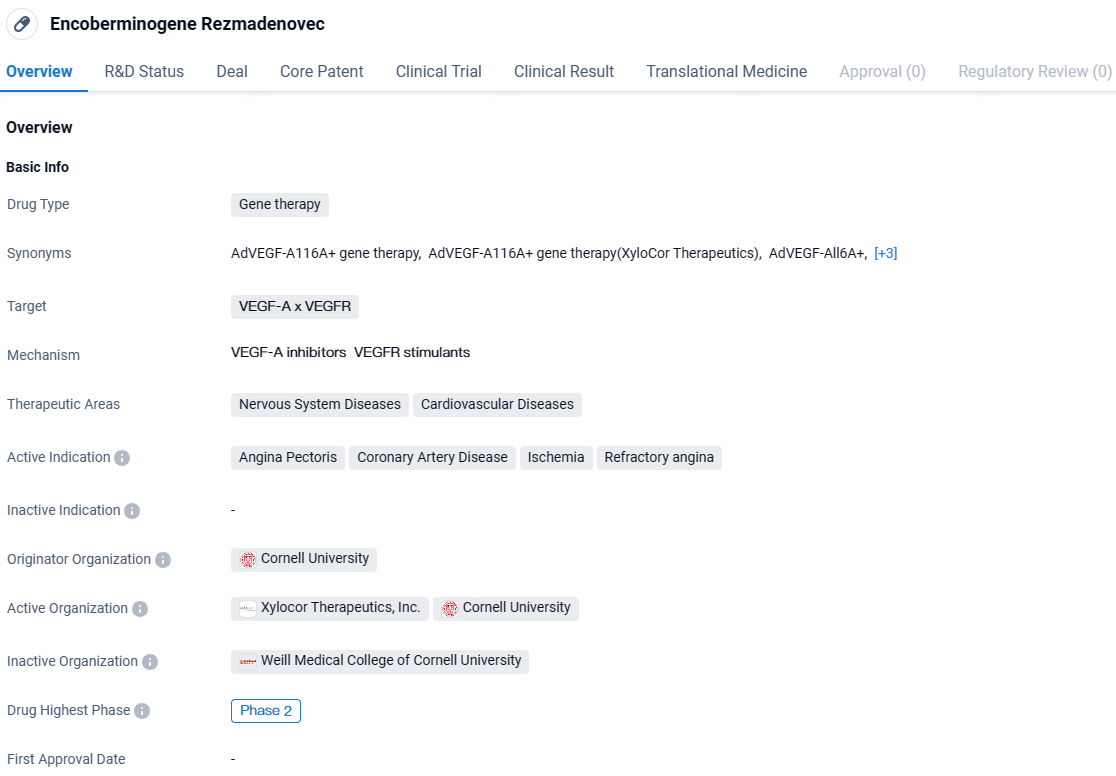
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
In the EXACT study, the efficacy of a single administration of gene therapy using XC001 was evaluated as an innovative treatment strategy for refractory angina, a severe and persistent condition affecting more than a million individuals in the U.S., with numbers on the rise.
XC001 aims to alleviate ischemic symptoms by inducing the formation of new cardiac blood vessels via the targeted expression of various vascular endothelial growth factor (VEGF) isoforms. During the Phase 2 stage of the EXACT study, a total of 32 participants diagnosed with class II-IV angina received the maximum dose of XC001 via a minimally invasive transepicardial procedure.
"The findings from the EXACT study indicate that XC001's angiogenic gene therapy could enhance cardiovascular health in patients with refractory angina who lack other treatment alternatives," stated Thomas Povsic, M.D., Ph.D., a Professor of Medicine at Duke University School of Medicine, and Kenta Nakamura, M.D., an Assistant Professor at the University of Washington, who were the principal investigators of the EXACT study.
Phase 2 findings confirmed XC001's significant potential to alter disease progression, reduce ischemia, and enhance life quality for heart disease patients with limited therapeutic options. Clinical improvements were consistently observed: longer exercise durations, reduced ischemic levels as determined by Positron Emission Tomography scans, and less frequent angina attacks.
Significantly, at the start of the study, 93% of participants experienced severe chest pain that severely restricted their everyday activities. Six months post-treatment, 43% of these patients reported no chest pain during regular activities. The XC001 VEGF gene therapy was well-received, with no serious drug-related or unexpected severe adverse events reported.
"The encouraging results from the EXACT trial signal an important advancement for individuals suffering from refractory angina and the broader cardiovascular field," remarked Al Gianchetti, President and CEO of XyloCor. "We are now planning our subsequent clinical study to further develop XC001 and continue to explore its significant medical potential for patients and their loved ones.”
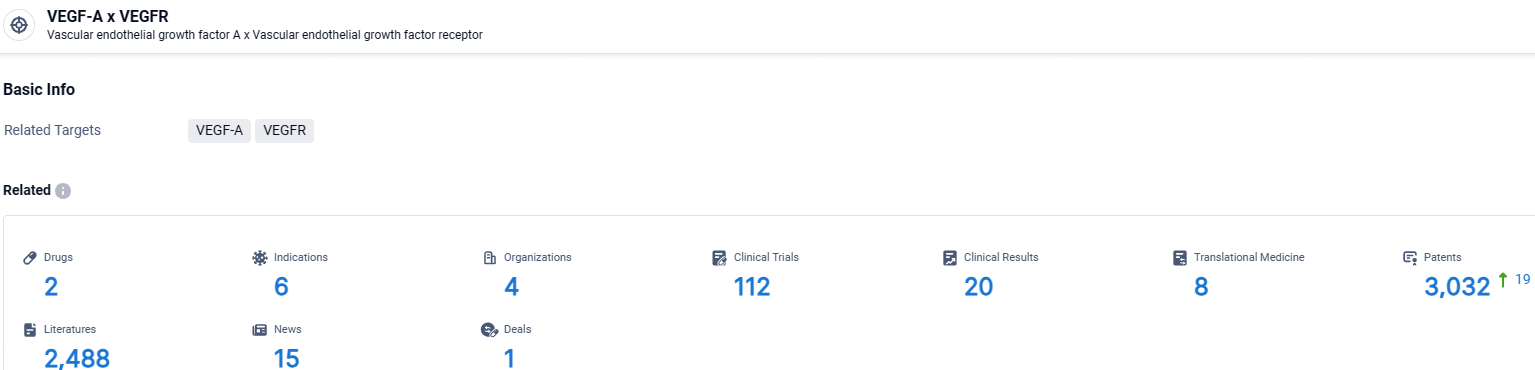
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 9, 2024, there are 2 investigational drugs for the VEGF-A and VEGFR target, including 6 indications, 4 R&D institutions involved, with related clinical trials reaching 112, and as many as 3032 patents.
XC001 is a gene therapy drug that targets VEGF-A x VEGFR and is being developed for the treatment of nervous system diseases and cardiovascular diseases. The active indications for this drug include angina pectoris, coronary artery disease, ischemia, and refractory angina. It is currently in Phase 2 of clinical development, indicating promising progress in its development.