Yunovia Receives MFDS IND Clearance for Phase 1 MAD Study of GLP-1 Small Molecule Agonist
Yunovia, a research and development arm of Ildong Pharmaceutical Group, revealed on the 26th that the Korean Ministry of Food and Drug Safety (MFDS) has approved the Investigational New Drug (IND) application for a Phase 1 Multiple Ascending Dose (MAD) study of ID110521156. This orally administered small molecule GLP-1 agonist is designed for the treatment of obesity and diabetes, following the successful conclusion of the Phase 1 Single Ascending Dose (SAD) study.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Phase 1 MAD study aims to assess the safety, tolerability, pharmacokinetics (PK), and pharmacodynamic (PD) profiles of ID110521156 through repeated dosing and incremental dosage increases.
In earlier investigations, Yunovia not only validated the drug's efficacy in insulin secretion and blood glucose regulation but also its superior tolerability compared to other drugs in its class during preclinical efficacy and toxicity reviews and the recently concluded Phase 1 SAD study.
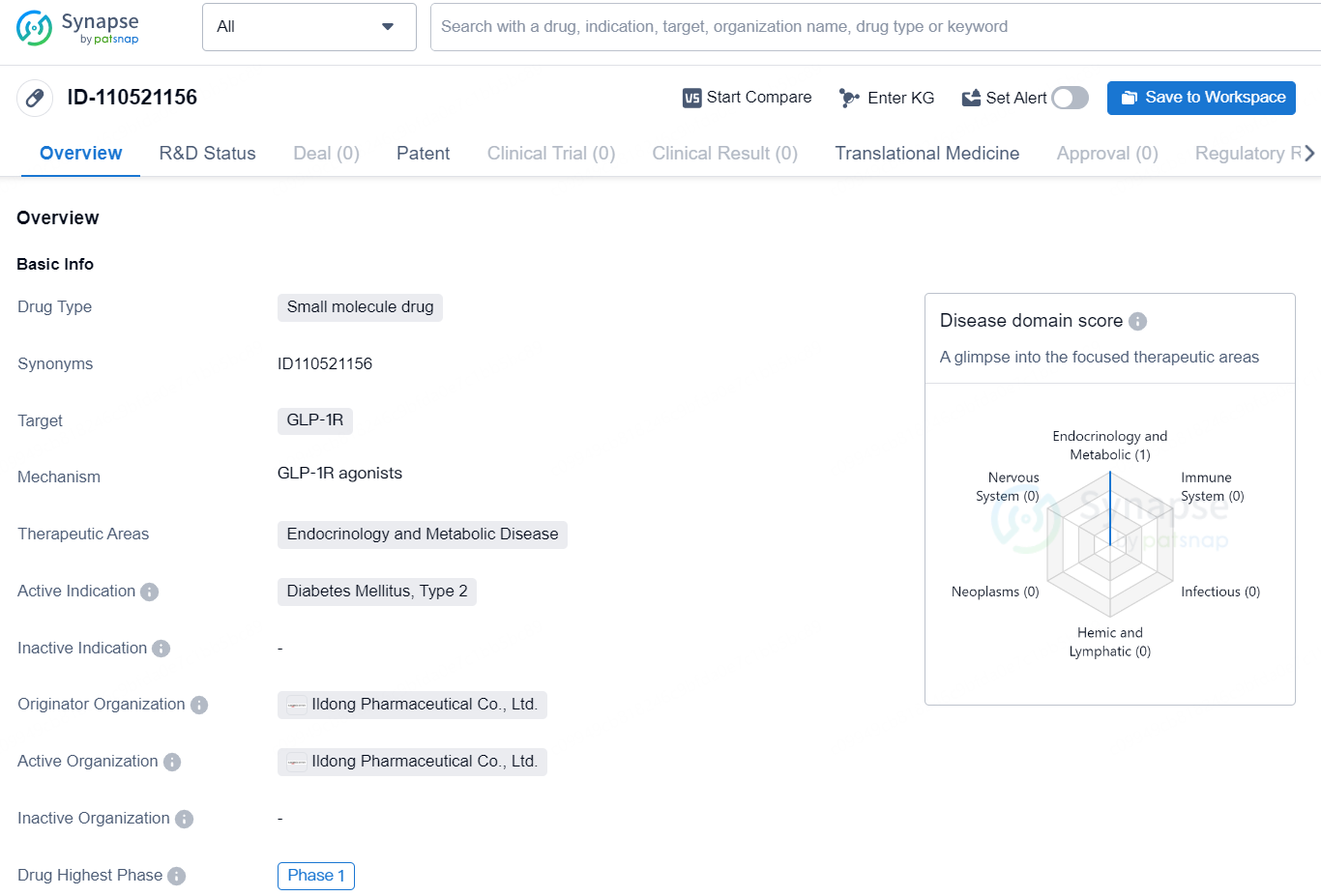
ID110521156 is a GLP-1 (glucagon-like peptide-1) receptor agonist that mimics the function of the GLP-1 hormone, which facilitates insulin synthesis and secretion, lowers blood glucose levels, regulates gastrointestinal motility, and suppresses appetite.
Given that ID110521156 is a small molecule compound, the Company aims to develop it as an orally administrable medication for diabetes and obesity, offering differentiated features like superior manufacturability and user convenience compared to peptide injections, which are currently the standard treatment.
A Yunovia spokesperson highlighted, “ID110521156 is the solitary small molecule-based investigational drug at a clinical stage among all GLP-1 receptor agonists in Korea. By global market standards, its development status is considered relatively advanced within the GLP-1 receptor agonist category.”
The spokesperson also mentioned, “We have been engaging with potential partner companies since the early development phase, incorporating their feedback on market needs into the design of our SAD and MAD studies. This ongoing engagement will guide the further development of ID110521156, with a focus on global out-licensing opportunities.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
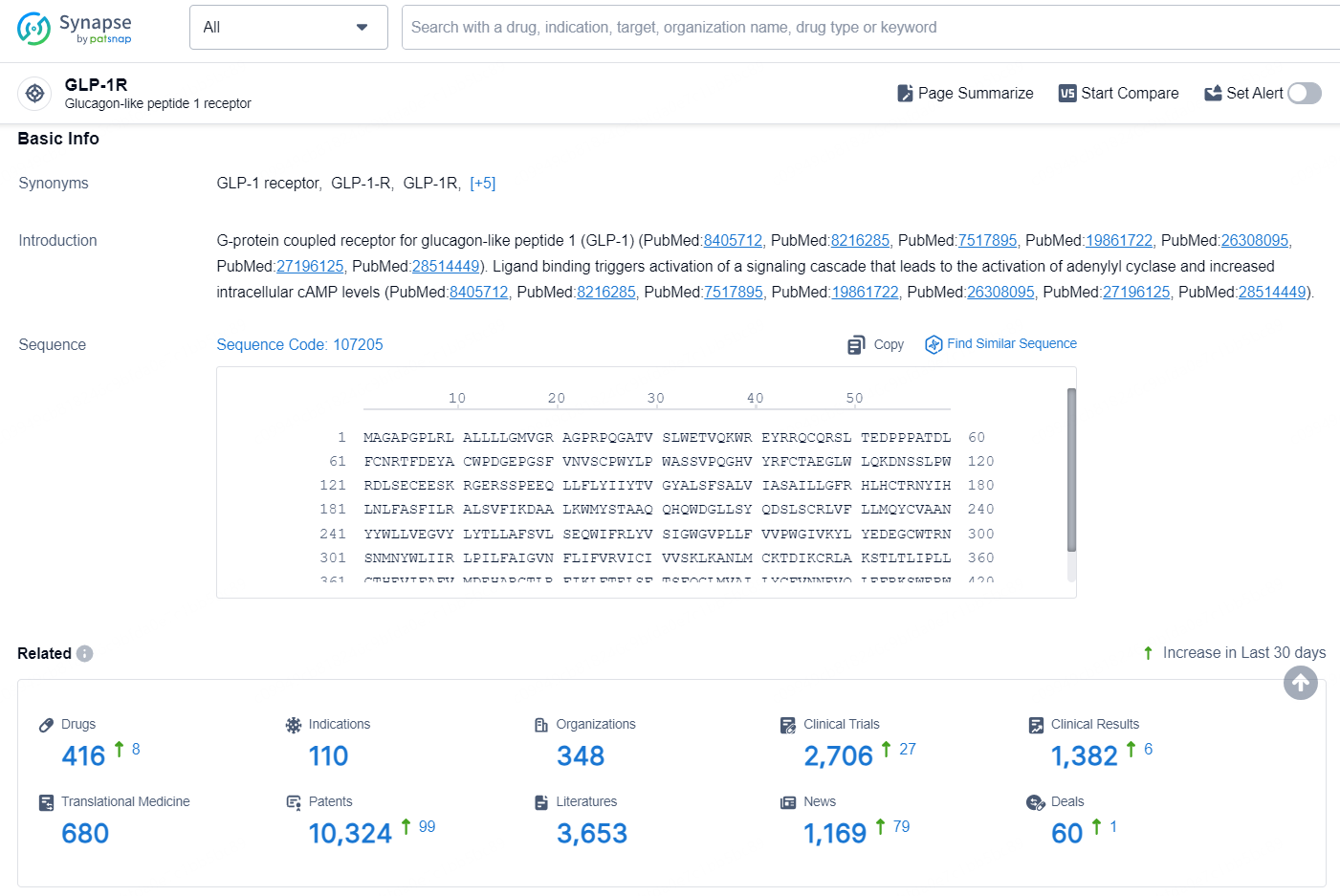
According to the data provided by the Synapse Database, As of August 30, 2024, there are 416 investigational drugs for the GLP-1R target, including 110 indications, 348 R&D institutions involved, with related clinical trials reaching 2706, and as many as 10324 patents.
ID110521156 is an orally available small molecule agonist of the GLP-1 receptor. Yunovia completed its Phase I SAD, including food effect study, in July 2024. ID110521156 was well tolerated and demonstrated potential as a once-daily drug with a sustained PK profile. The company plans to collect PD data, including continuous glucose monitoring and body weight changes, as exploratory endpoints from a 4-week MAD study.