Tonix Pharmaceuticals and Bilthoven Biologicals Collaborate to Develop TNX-801 Mpox Vaccine
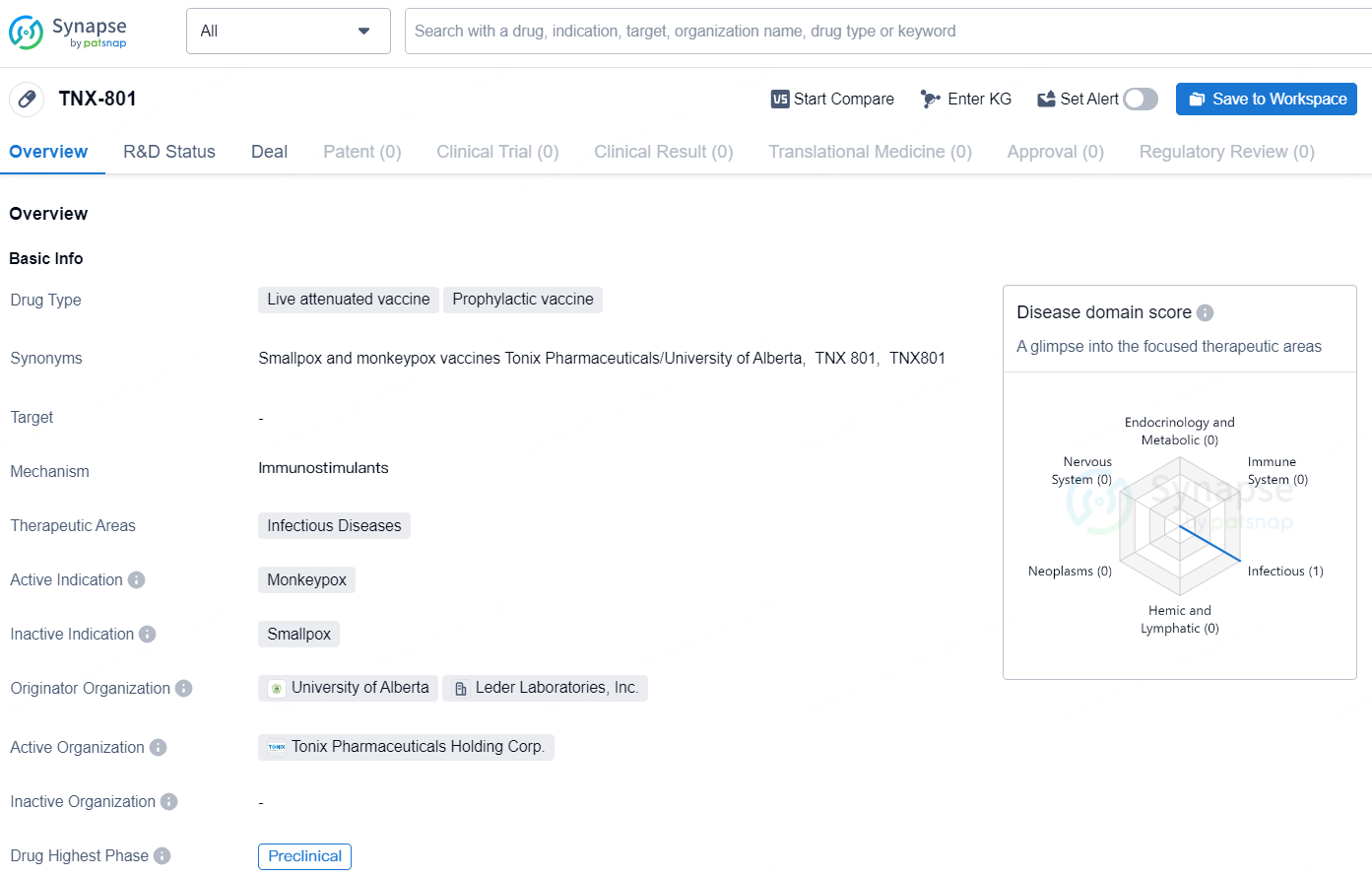
Tonix Pharmaceuticals Holding Corp. (Nasdaq: TNXP) (Tonix), a comprehensive biopharmaceutical firm with commercialized products and a robust pipeline of development candidates, and Bilthoven Biologicals (BBio), belonging to the Cyrus Poonawalla Group, the world’s largest vaccine producer which encompasses the Serum Institute of India, have jointly announced a partnership to further the development of TNX-801, Tonix’s vaccine candidate for mpox. TNX-801 (recombinant horsepox virus) is an attenuated, live replicating virus vaccine derived from horsepox, currently in preclinical stages, aimed at preventing mpox and smallpox.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
TNX-801 utilizes a technology that could serve as a viral vector platform, facilitating the development of recombinant variants aimed at combating different infectious diseases. BBio, a globally recognized vaccine manufacturer, produces both preventative and therapeutic vaccines. BBio has been chosen by the European Union to participate in its 'ever warm' vaccine manufacturing initiative, focusing on pandemic preparedness.
In animal studies, TNX-801 has exhibited immune protection with superior tolerability compared to 20th-century vaccinia virus-based vaccines. Preclinical research demonstrated that TNX-801 safeguarded non-human primates from a lethal intratracheal exposure to Clade 1 monkeypox virus. A single dose vaccination of TNX-801 effectively prevented clinical symptoms and lesions and reduced viral shedding in the oral and pulmonary regions of non-human primates. These results align with the concept of mucosal immunity, indicating potential for blocking onward transmission.
On August 14, 2024, the World Health Organization (WHO) declared the surge in mpox cases across several African nations a public health emergency of international concern, marking the second such declaration in response to an mpox outbreak within two years. The current outbreak has been traced to Clade 1 monkeypox virus, whereas the 2022 outbreak was attributed to Clade 2 monkeypox virus. The Clade 2 mpox outbreak starting in 2022 has infected over 90,000 individuals in regions previously non-endemic to mpox, including Europe and the U.S. The 2022 spread of Clade 2b mpox highlights the pandemic potential of the disease. In Central African countries such as the Democratic Republic of the Congo, mpox remains endemic, with Clade 1 exhibiting up to a 10% mortality rate.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
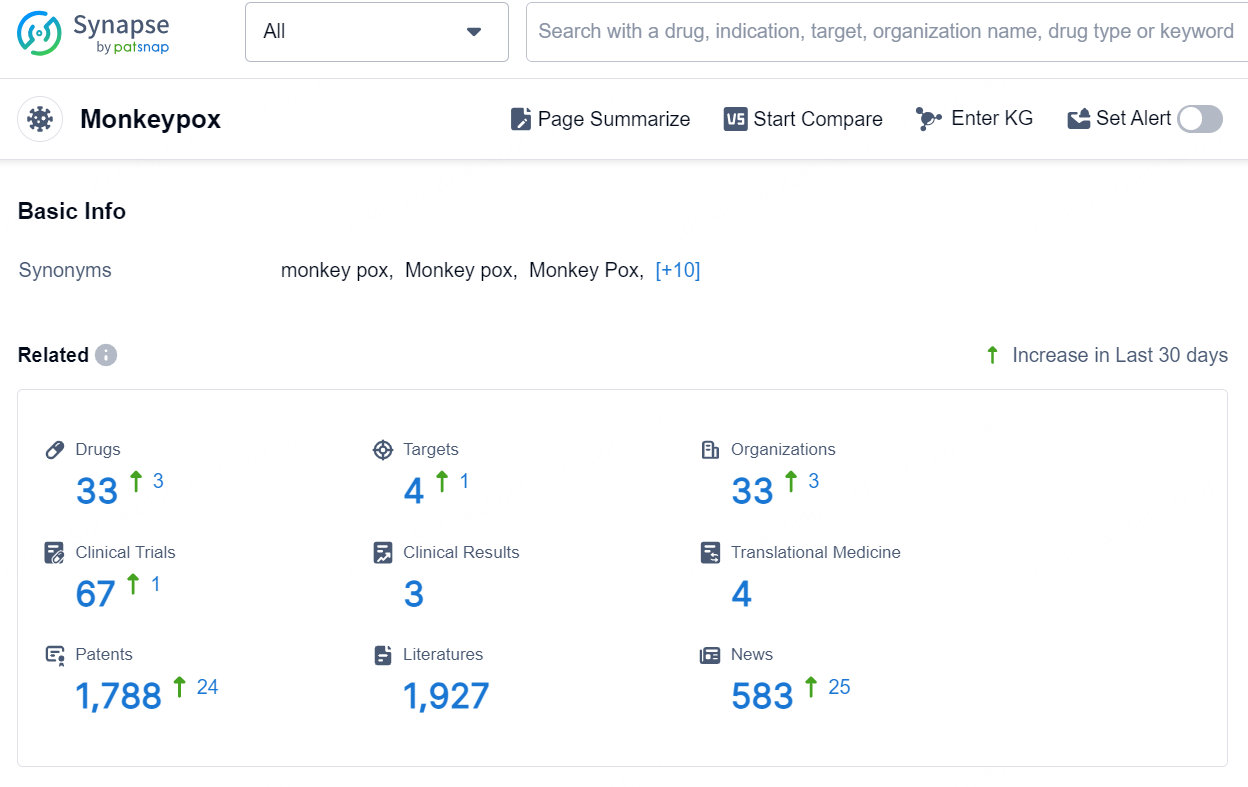
According to the data provided by the Synapse Database, As of August 29, 2024, there are 33 investigational drugs for the monkeypox, including 4 targets, 33 R&D institutions involved, with related clinical trials reaching 67, and as many as 1788 patents.
Ustekinumab Biosimilar (Celltrion) is a biosimilar drug classified as a monoclonal antibody. It is designed to target IL-12 and IL-23 and is intended to treat a range of therapeutic areas including immune system diseases, infectious diseases, digestive system disorders, and skin and musculoskeletal diseases. The drug is indicated for the treatment of Crohn's disease (active moderate and severe), psoriatic arthritis, ulcerative colitis, and plaque psoriasis.