EU Commission Approves RYBREVANT® (Amivantamab) with Chemotherapy for Treatment-Resistant Advanced EGFR-Mutated NSCLC in Adults
Janssen-Cilag International NV, part of Johnson & Johnson, announced that the European Commission (EC) has approved a Type II indication extension for RYBREVANT®▼ (amivantamab). This extension allows the drug to be used alongside chemotherapy (carboplatin and pemetrexed) for treating adults with advanced non-small cell lung cancer (NSCLC) characterized by epidermal growth factor receptor (EGFR) Exon 19 deletions (ex19del) or Exon 21 L858R substitution (L858R) mutations. This indication is specifically for patients who have experienced failure with prior therapies, including an EGFR tyrosine kinase inhibitor (TKI).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"In recent years, significant advancements have been made in lung cancer treatment; however, resistance to current therapies remains a significant challenge for those with advanced or metastatic non-small cell lung cancer harboring EGFR mutations. This highlights the need for continual innovation," stated Antonio Passaro, M.D., Ph.D., a Medical Oncologist in the Division of Thoracic Oncology at the European Institute of Oncology in Milan, Italy. "Integrating the bispecific antibody amivantamab with chemotherapy provides a crucial new treatment choice for patients with EGFR ex19del or L858R mutations, who progress on or after osimertinib. This combination has now established a new benchmark in overall response rate while reducing the risk of disease progression or death by over 50% compared to chemotherapy alone. It also showed significant gains in intracranial progression-free survival."
"The combination approval of amivantamab and chemotherapy fills a significant gap for patients whose disease has worsened after EGFR TKI treatment, who previously had limited options," mentioned Henar Hevia, Ph.D., Senior Director and EMEA Therapeutic Area Lead for Oncology at Johnson & Johnson Innovative Medicine. "This achievement underscores the important role of precision medicine in delivering better outcomes for lung cancer patients."
The expanded indication for amivantamab is supported by results from the Phase 3 MARIPOSA-2 study (NCT04988295), assessing the efficacy and safety of amivantamab combined with chemotherapy in patients with locally-advanced or metastatic EGFR ex19del or L858R substitution NSCLC, who had disease progression post-osimertinib treatment. The amivantamab plus chemotherapy group achieved its primary endpoint, showing a 52% reduction in the risk of disease progression or death compared to chemotherapy alone, with a median progression-free survival (PFS) of 6.3 months versus 4.2 months (hazard ratio [HR]=0.48; 95% confidence interval [CI], 0.36–0.64; P<0.001). Moreover, the combination exhibited an objective response rate (ORR) of 64%, in contrast with 36% for chemotherapy alone."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
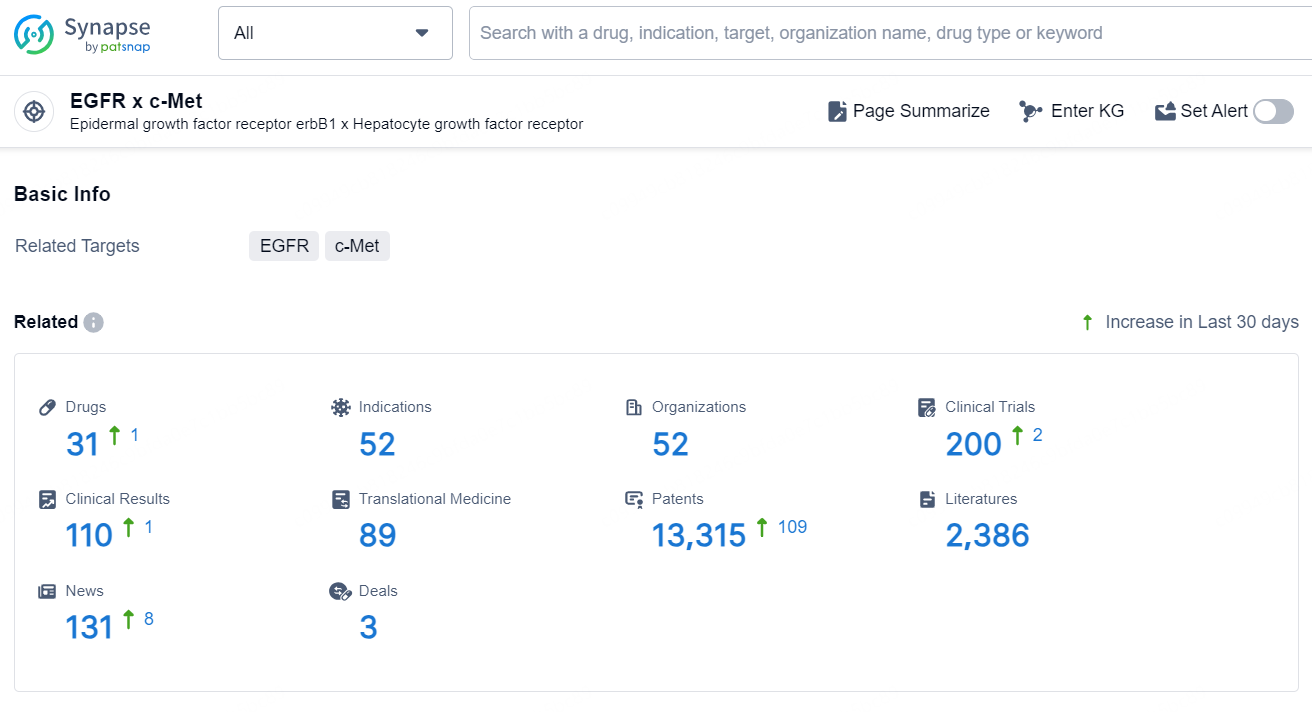
According to the data provided by the Synapse Database, As of August 29, 2024, there are 31 investigational drugs for the EGFR x c-Met targets, including 52 indications, 52 R&D institutions involved, with related clinical trials reaching 200, and as many as 13315 patents.
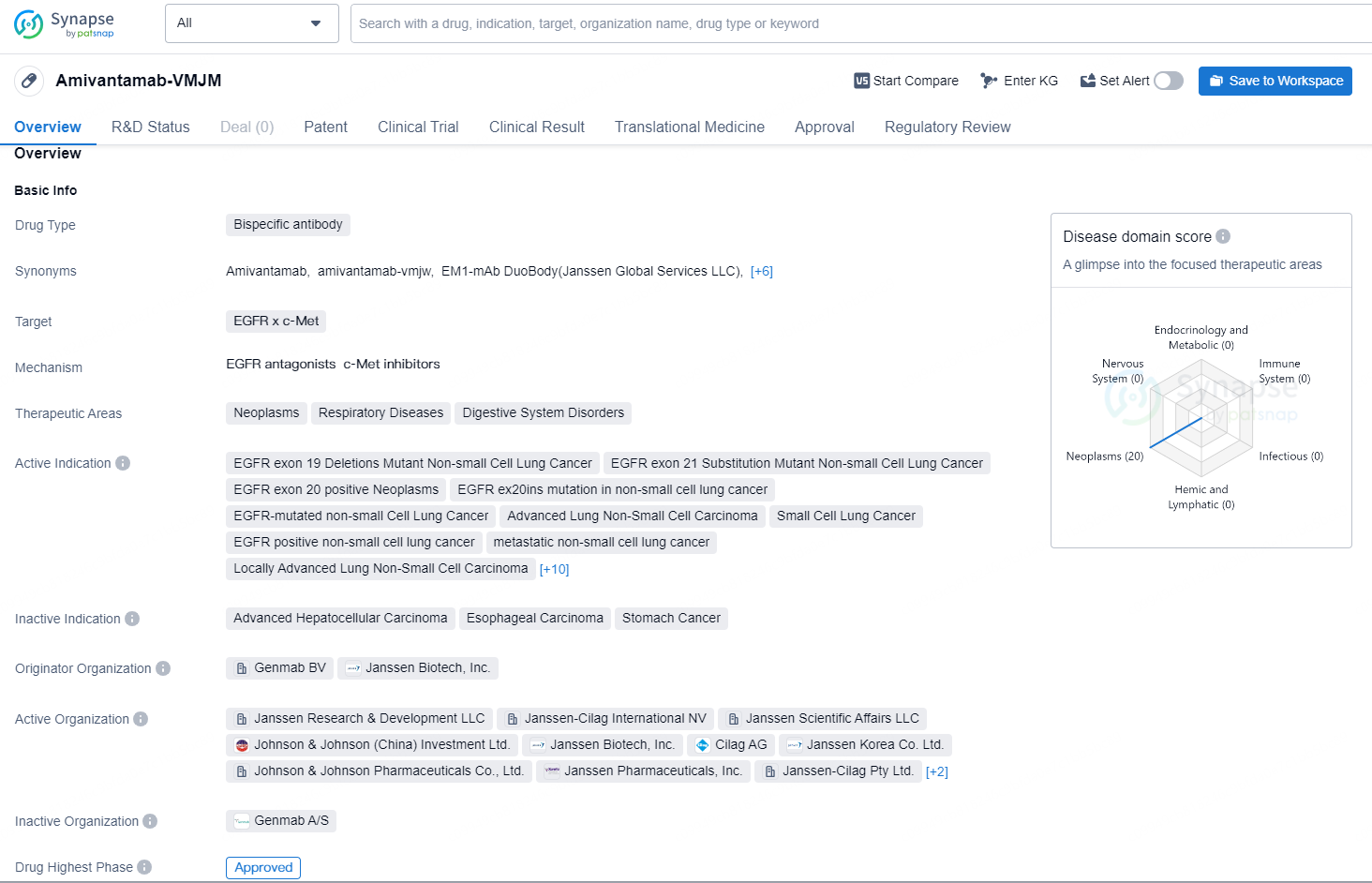
Amivantamab-VMJM is a bispecific antibody designed to target EGFR (epidermal growth factor receptor) and c-Met, making it suitable for the treatment of various neoplasms, respiratory diseases, and digestive system disorders. The drug is indicated for specific mutations and types of non-small cell lung cancer, including EGFR exon 19 deletions, exon 21 substitutions, and exon 20 insertions. It is also indicated for metastatic and locally advanced lung non-small cell carcinoma, small cell lung cancer, and other related conditions such as recurrent non-small cell lung cancer, secondary malignant neoplasm of the lung, and squamous cell carcinoma of the head and neck. In addition, the drug is indicated for gastrointestinal neoplasms, colorectal cancer, and advanced malignant solid neoplasms.