Request Demo
Last update 27 Nov 2025
Iopromide
Last update 27 Nov 2025
Overview
Basic Info
Drug Type Contrast agent, Small molecule drug |
Synonyms Iopromide (JAN/USP/INN), N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-5-[(methoxyacetyl)amino]-N-methylisophthalamide, 碘普胺 + [7] |
Action antagonists, enhancers |
Mechanism M1 receptor antagonists(Muscarinic acetylcholine receptor M1 antagonists), M3 receptor antagonists(Muscarinic acetylcholine receptor M3 antagonists), Radiography enhancers |
Therapeutic Areas |
Active Indication |
Inactive Indication- |
Originator Organization- |
Active Organization |
Inactive Organization |
License Organization- |
Drug Highest PhaseApproved |
First Approval Date Australia (12 Sep 1991), |
Regulation- |
Login to view timeline
Structure/Sequence
Molecular FormulaC18H24I3N3O8 |
InChIKeyDGAIEPBNLOQYER-UHFFFAOYSA-N |
CAS Registry73334-07-3 |
Related
15
Clinical Trials associated with IopromideNCT03631771
A Multicenter, Prospective Study to Evaluate the Risk of Hypothyroidism in Pediatric Patients Administered Intravascular Iohexol, Iodixanol, Iopromide, or Ioversol. THYROPED.
This is a Phase 4 multicenter prospective study to estimate the proportion of patients aged birth to 3 years who develop hypothyroidism within 6 months after receiving intravascular iohexol (Omnipaque™), iodixanol (Visipaque™), iopromide (Ultravist®), or ioversol (Optiray®) as the iodinated contrast medium (ICM) for clinical evaluation during an ICM-enhanced diagnostic imaging procedure.
Start Date01 Mar 2022 |
Sponsor / Collaborator |
NCT04605471
Safety Profile of Ultravist in Children and Elderly (UV Age)
Ultravist is an iodine-based contrast agent that helps to make medical imaging scans clearer. It is also called iopromide, and it is available for doctors to give patients before they have scans. Even after a treatment or substance has been approved for use, researchers continue to study it to learn more about its safety.
Researchers have done studies on the safety of Ultravist, but they want to learn more about specific medical problems called hypersensitivity reactions (HSRs). These are undesirable immune system reactions to the study drug. In this study, the researchers will compare the risk of HSRs in children and in the elderly to the risk of HSRs in middle aged adults.
The researchers will look at information about medical problems that happened in people in 4 other studies. These studies are called PMS I, IMAGE, TRUST, and Ultravist in CT. A total of about 139,000 people will be included in this study.
All of the people in the earlier 4 studies received Ultravist as an injection into the vein or artery before having a scan. In this study, the researchers will compare the number of children, middle aged adults, and elderly patients who had HSRs after receiving Ultravist.
Researchers have done studies on the safety of Ultravist, but they want to learn more about specific medical problems called hypersensitivity reactions (HSRs). These are undesirable immune system reactions to the study drug. In this study, the researchers will compare the risk of HSRs in children and in the elderly to the risk of HSRs in middle aged adults.
The researchers will look at information about medical problems that happened in people in 4 other studies. These studies are called PMS I, IMAGE, TRUST, and Ultravist in CT. A total of about 139,000 people will be included in this study.
All of the people in the earlier 4 studies received Ultravist as an injection into the vein or artery before having a scan. In this study, the researchers will compare the number of children, middle aged adults, and elderly patients who had HSRs after receiving Ultravist.
Start Date31 Oct 2020 |
Sponsor / Collaborator |
NCT03622801
Risk of Anaphylactoid Reactions of Iopromide After Intra-arterial Administration
Iopromide (trade name is Ultravist) is on the market for more than 30 years and has been used more than 250 million times as X-ray contrast medium for patients. It is known that Iopromide may cause allergy-like reactions after being injected. With this study researchers want to find out, if the risk of severe allergy-like reactions is lower, when Iopromide will be injected into an artery, compared to the risk after an injection of Iopromid into a vein. To find this out data from four trials on Iopromide that are already completed will be combined and newly analyzed. The database used for this analysis will contain data from more than 150,000 patients.
Start Date12 Oct 2018 |
Sponsor / Collaborator |
100 Clinical Results associated with Iopromide
Login to view more data
100 Translational Medicine associated with Iopromide
Login to view more data
100 Patents (Medical) associated with Iopromide
Login to view more data
1,423
Literatures (Medical) associated with Iopromide19 Oct 2025·PHYSICS IN MEDICINE AND BIOLOGY
Metal artifact reduction and contrast agent performance as a function of source spectral shape
Article
Author: Aubrey, Jacob D ; Perkinson, Emmett P ; Bonitatibus, Peter J ; Wang, Ge
Abstract:
Objective. Empirically map the x-ray source of a Medipix All Resolution Scanner (MARS) photon counting CT (PCCT) with Cu and Sn pre-filters, assess metal artifact reduction (MAR) capabilities of these pre-filters, and measure pre-filtration impact on contrast performance (CP) of FDA approved iopromide and experimental tantalum oxide nanoparticles (TaOx NPs). Approach. The x-ray source of a MARS-PCCT system was empirically mapped with no pre-filtration, seven Cu filters (0.3–2.1 mm), and seven Sn filters (0.15–1.05 mm). A phantom with inserts containing water, lipid, iopromide, TaOx NPs, and metal was scanned with no pre-filtration and Cu and Sn pre-filters. Insert noise, signal, contrast-to-pooled-noise ratio (CPNR), and a fast Fourier transform artifact metric (FFTAM) were calculated for each filter to quantify MAR and CP. Main results. Thick filters for Cu and Sn shifted mean energy of the unfiltered x-ray source (47.9 keV) by 19.2 and 23.4 keV, respectively. Thick filtration, 2.1 mm Cu and 0.6 mm Sn, greatly reduced noise (up to 74%) and FFTAM (up to 71%) for all inserts and energy bins. Thin filtration, 0.3 mm Cu and 0.15 mm Sn, also reduced noise (up to 47%) and FFTAM (up to 41%). In most cases, iopromide lost significant contrast (up to 50%). TaOx NPs also lost contrast, though to a lesser extent (up to 38%). Pre-filtration improved image efficacy (i.e. CPNR), especially for TaOx (up to 61%). Significance. By empirically mapping the source spectrum of a MARS-PCCT system with pre-filters, valuable information was gathered about photon flux distribution and detector artifacts; these findings will prove insightful for applications such as energy binning for effective material decomposition. Furthermore, this information will potentially guide clinical MAR development, most notably for the MARS Extremity 5 × 120 recently deployed for first-in-human trials. Lastly, TaOx NPs were shown to be more compatible than iopromide with spectral shaping.
01 Sep 2025·JOURNAL OF PHARMACEUTICAL AND BIOMEDICAL ANALYSIS
Simultaneous determination of three iodinated contrast media in human plasma by LC/MS-MS and its clinical application
Article
Author: Wu, Xiaofei ; Wang, Hongyun ; Wang, Yaoyao ; Cai, Jianfang ; Liu, Shupeng
Iodinated contrast media (ICM), including iohexol, iopromide and iodixanol, have significantly enhanced diagnostic accuracy, particularly in cardiovascular and cerebrovascular diseases. The three ICM is tailored to specific patient needs and diagnostic contexts. However, excessive doses of ICM may induce adverse reactions, including nephrotoxicity. Accurate measurement of ICM concentration is essential for reducing renal burden and ensuring patient safety. Current methods for quantifying ICM are often limited to single-analyte detection (especially iohexol), which is inefficient for clinical practice. Therefore, this method aims to develop and validate a rapid, generic LC-MS/MS method for simultaneously quantifying iohexol, iopromide, and iodixanol in human plasma to improve patient management and prognosis. An LC-MS/MS-based approach was established utilizing an C18 column coupled with a Xevo TQ-S triple quadrupole mass spectrometer. Sample preparation involved protein precipitation, while chromatographic separation was achieved using acetonitrile and 0.1 % formic acid in water. The developed LC-MS/MS method achieved satisfactory separation of the three ICM within 1.5 min. Calibration curves for iohexol, iopromide, and iodixanol showed good linearity in 5.00-5000, 5.00-5000 and 10.0 ∼ 10000 µg/mL, respectively. The method was effectively applied to analyze 71 plasma samples from 9 patients, highlighting its clinical utility. A rapid and generic LC-MS/MS method streamlines sample preparation and standardizes laboratory workflows, making it suitable for clinical bio-samples.
01 Aug 2025·JOURNAL OF HAZARDOUS MATERIALS
Evaluation of micropollutant removal from artificially recharged water using activated carbon
Article
Author: Šváb, Marek ; Vorokhta, Maryna ; Švábová, Martina ; Pohořelý, Michael
Pilot scale micropollutant removal onto three types of granular activated carbon was tested over a period of 25 months. The columns were operated in a mode as similar as possible to the functioning of the main waterworks with the emphasis on minimizing the washing of the activated carbon layers. Sampling was performed monthly and a total of 222 micropollutants were evaluated. The columns were located at a waterworks in central Bohemia (Czech Republic). Real influent water from the river Jizera after artificial recharge and sand filtration was used. The aim of this study was to verify whether the process of micropollutant removal was suitable under the operating conditions at the waterworks. Clear seasonal patterns of micropollutant concentrations in the influent were detected. All three columns were able to effectively remove or substantially decrease the concentration of most micropollutants. The best performance was shown for the activated carbon Aquasorb 5005, which had the highest surface area and a well developed porous structure with the highest proportion of mesopores. The data were evaluated in order to identify micropollutant parameters important for their removal, and the pH-dependent octanol/water partition coefficient was identified as the key parameter. Other important parameters were charge and polar surface area of the micropollutant molecules.
6
News (Medical) associated with Iopromide22 Sep 2023
Bayer and Hologic say contrast-enhanced mammography can be used alongside traditional scans, with injectable agents helping visualize the blood vessels leading in and out of a breast tumor.
Bayer and Hologic have teamed up with the goal of offering clearer breast cancer scans as a package deal—combining the former’s imaging contrast agents with the latter’s mammography hardware.
The two companies say that the emerging field of contrast-enhanced mammography, or CEM, can be used alongside traditional scans—with the addition of contrast agents highlighting the flow of blood and helping to visualize the vessels leading in and out of a tumor.
It can be a helpful option after an inconclusive reading from a previous breast scan, or during the planning stages before tumor surgery, according to Bayer and Hologic. CEM can also be used to provide a somewhat similar, but less expensive, image compared to contrast-enhanced MRI, or when those machines are unavailable or unsafe for the patient.
The partnership will focus on Europe, Canada and the Asia-Pacific region, with plans to provide hands-on training to radiologists and clinicians on the administration of injectable contrast agents during digital mammography.
“Over the past several years, we’ve seen an increased interest in contrast-enhanced mammography as an additional diagnostic modality,” Tanja Brycker, Hologic’s VP of strategic development for breast and skeletal health, said in a statement. “Our partnership with Bayer will enable clinicians around the world to offer CEM as part of the breast cancer diagnostic workflow.”
In January of this year, Bayer received European approval for its Ultravist contrast agent for use in CEM. The iodine-based Ultravist has previously been used in X-ray, angiography and CT scans, and according to Bayer it has been used in more than 100 countries for scans of the head, chest, heart, abdomen and liver. The agent also received a CEM approval from the FDA in June.
Diagnostic Reagents
26 Jun 2023
Ultravist-300, -370 is now the only contrast agent in the U.S. indicated to visualize known or suspected lesions of the breast in adults, as an adjunct to mammography and/or ultrasound
Headquarters of Bayer Pharmaceuticals in Berlin-Wedding. (Credit: Fridolin freudenfett/Wikipedia)
Bayer announced today that Ultravist® (iopromide)-300, -370, its iodine-based contrast agent, has been approved by the U.S. Food and Drug Administration (FDA) for contrast-enhanced mammography (CEM) – making it the only contrast agent approved for this indication. The product can be used to visualize known or suspected lesions of the breast in adults, as an adjunct to mammography and/or ultrasound. CEM is an emerging modality that combines digital mammography with the administration of a contrast agent, such as Ultravist, to help identify breast lesions.
The new FDA approved indication aligns with the recent increased focus on supplemental imaging needs for women at a higher risk for breast cancer, which may include the 40-50% of U.S. women older than 40 with dense breasts.
“The approval of Ultravist-300 and -370 in contrast-enhanced mammography gives physicians a new imaging option where conventional mammography might not be enough,” said Dr. Konstanze Diefenbach, Head of Radiology Research and Development, Bayer. “We are pleased to be able to offer additional options for breast imaging to healthcare professionals, as we aim to support them in their role of providing clear answers from diagnosis to care for patients.”
The approval expands upon Bayer’s focus on breast imaging, with a portfolio that also includes Gadavist ® (gadobutrol) injection, a gadolinium-based contrast agent approved for use with MRI (Magnetic Resonance Imaging) to assess the presence and extent of malignant breast disease in adult patients. In 2019, the MEDRAD® Stellant FLEX Computed Tomography (CT) Injection System with Certegra® Workstation was also cleared in the U.S. for use in CEM. Through the use of iodine-based x-ray contrast agents, CEM can allow for better visualization of abnormalities in breast tissue that may not be visible with standard mammography.
Source: Company Press Release
Drug Approval
27 Feb 2023
Leverkusen, February 28, 2023
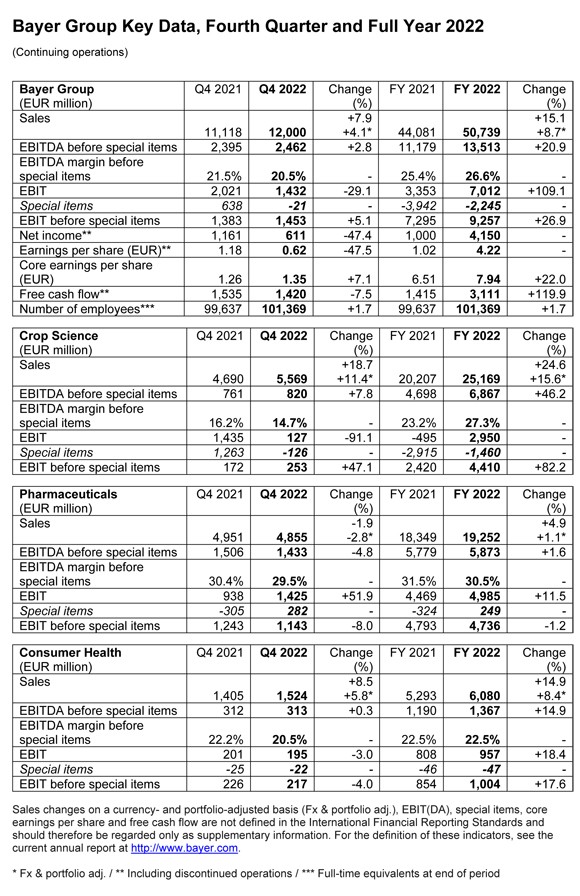
– The Bayer Group achieved strong growth last year, posting significantly higher sales and earnings. “2022 was a very successful year for Bayer despite the challenging environment. We were able to deliver, even during these difficult times, and met the upgraded financial targets we set in August,” said Werner Baumann, Chairman of the Board of Management, at the company’s Financial News Conference on Tuesday. The company is active in the right fields, he said. “Health and nutrition are fundamental human needs. Our vision of
Health for all, hunger for none
is and will remain vitally important, especially in times like these.”
Group sales came in at 50.739 billion euros in 2022, up 8.7 percent on a currency- and portfolio-adjusted basis (Fx & portfolio adj.). EBITDA before special items rose by 20.9 percent to 13.513 billion euros, and included a positive currency effect of 429 million euros (2021: negative currency effect of 507 million euros). The EBITDA margin before special items increased to 26.6 percent (2021: 25.4 percent). EBIT amounted to 7.012 billion euros, and was therefore more than twice as high as in the previous year. Net income came in at 4.150 billion euros (2021: 1.000 billion euros), while core earnings per share rose by 22.0 percent to 7.94 euros.
Free cash flow more than doubled against the prior year, rising to 3.111 billion euros. Net financial debt amounted to 31.809 billion euros as of December 31, 2022, down 4.0 percent from year-end 2021. Cash inflows from operating activities and the sale of the Environmental Science Professional business were partially offset by cash outflows for dividends and negative currency effects.
“These are very good results. Together with the Supervisory Board, we are therefore proposing a dividend of 2.40 euros to the Annual Stockholders’ Meeting. This represents a 20 percent increase compared to the previous year,” said Chief Financial Officer Wolfgang Nickl. With 982.42 million shares entitled to the dividend, the company is therefore set to distribute a total of 2.358 billion euros to stockholders for fiscal 2022 (fiscal 2021: 1.965 billion euros).
Crop Science achieves record sales and industry-leading margin
Sales in the agricultural business (Crop Science) advanced by 15.6 percent (Fx & portfolio adj.) to a record 25.169 billion euros, with business up in all regions. Growth was strongest at Herbicides (Fx & portfolio adj. 43.9 percent), which saw sales rise in Latin and North America and in Europe/Middle East/Africa in particular thanks to higher prices, as supply for glyphosate-based products was tight. Sales at Corn Seed & Traits rose 8.8 percent (Fx & portfolio adj.) as the division increased its market share. Price increases in all regions more than offset a decrease in acreages in North America and lower license revenues. Sales at Fungicides were up 5.2 percent (Fx & portfolio adj.), with higher prices in the Latin America and Europe/Middle East/Africa regions in particular more than offsetting a decline in volumes in North America. Sales at Soybean Seed & Traits were level with the prior year, with business growing in Latin America but declining in North America due to lower volumes.
EBITDA before special items at Crop Science advanced by 46.2 percent to 6.867 billion euros, mainly due to the significant increase in sales. Earnings also benefited from contributions from ongoing efficiency programs and a positive currency effect of 284 million euros (2021: negative currency effect of 387 million euros). By contrast, earnings were mainly diminished by an increase in the cost of goods sold, which was primarily due to high inflation. The EBITDA margin before special items increased by 4.1 percentage points to an industry-leading 27.3 percent.
Pharmaceuticals benefits from new products and Eylea™
Sales of prescription medicines (Pharmaceuticals) increased by 1.1 percent (Fx & portfolio adj.) to 19.252 billion euros. Over half a billion euros in sales came from the division’s new products: the cancer drug Nubeqa™ and Kerendia™ for the treatment of chronic kidney disease associated with type 2 diabetes. Sales of Nubeqa™, for instance, almost doubled year on year. Strong growth was also recorded for the ophthalmology drug Eylea™ (Fx & portfolio adj. plus 9.2 percent) and in the radiology business, which includes the Gadovist™ (Fx & portfolio adj. plus 9.0 percent) and Ultravist™ (Fx & portfolio adj. plus 17.5 percent) product lines. This was partially offset by declines due to factors such as additional tender procedures in China, especially for the oral anticoagulant Xarelto™ (Fx & portfolio adj. minus 5.8 percent) and the cancer drug Nexavar™ (Fx & portfolio adj. minus 41.2 percent). Xarelto™ sales were also impacted by price pressure in the United Kingdom and the expiration of the patent in Brazil.
EBITDA before special items at Pharmaceuticals rose by 1.6 percent to 5.873 billion euros, benefiting from the increase in sales and, to a lesser extent, from income from the sale of non-core businesses. Earnings were diminished by investments in marketing new products and higher research and development expenses for platform technologies and projects in advanced clinical development. In addition, higher costs due to a sharp increase in procurement prices had a negative effect. There was a positive currency effect of 9 million euros (2021: negative currency effect of 77 million euros). The EBITDA margin before special items declined by 1.0 percentage points to 30.5 percent.
Consumer Health grows business in all regions and categories
Sales of self-care products (Consumer Health) rose by 8.4 percent (Fx & portfolio adj.) to 6.080 billion euros, with growth in all regions and categories against a strong prior year. The Allergy & Cold business registered the strongest gains, with sales up by a significant 21.5 percent following continuously elevated cold incidence rates and the launch of the Astepro™ antihistamine nasal spray in the United States. The Dermatology category also achieved double-digit percentage growth (Fx & portfolio adj. 10.5 percent), in part due to higher demand for Bepanthen™. After a very strong prior year, the Nutritionals business also registered an increase in sales (Fx & portfolio adj. 1.0 percent).
EBITDA before special items at Consumer Health rose by 14.9 percent to 1.367 billion euros following the substantial increase in sales, strong contributions from productivity programs and active price management. The division achieved this growth in a business environment that was impacted by significant inflation-related cost increases while also making large investments in the launch of innovative products, especially for Astepro™. There was a positive currency effect of 85 million euros (2021: negative currency effect of 39 million euros). The EBITDA margin before special items came in at 22.5 percent, matching the prior-year figure.
Outlook: Further growth in sales – Earnings below prior year due to inflation and price factors
“Following two consecutive years of high single-digit percentage growth rates, we expect our business to remain at a high level and grow by two to three percent in 2023 on a currency- and portfolio-adjusted basis,” said Baumann. The company anticipates lower prices for agricultural herbicides as well as for some of its established pharmaceutical products. Projected sales growth in the other parts of the portfolio and from new products are expected to have a positive impact. As regards earnings in 2023, growth-driven margin contributions and positive effects from ongoing efficiency programs will not be sufficient to offset the anticipated decline in prices as well as high inflation-driven cost increases, which are expected to continue.
On a currency-adjusted basis (i.e. based on the average monthly exchange rates in 2022), Bayer expects to generate sales of 51 billion to 52 billion euros in 2023. The company anticipates EBITDA before special items of 12.5 billion to 13.0 billion on a currency-adjusted basis (Fx adj.). It forecasts core earnings per share of 7.20 to 7.40 euros (Fx adj.) and free cash flow of approximately 3.0 billion euros (Fx adj.). Net financial debt as of year-end 2023 is expected to amount to 32 billion to 33 billion euros (Fx adj.).
With respect to the divisions, Bayer anticipates sales growth (Fx & portfolio adj.) of around 3 percent at Crop Science, approximately 1 percent at Pharmaceuticals, and roughly 5 percent at Consumer Health. The company also expects the EBITDA margin before special items (Fx adj.) to come in at 25 to 26 percent at Crop Science, above 29 percent at Pharmaceuticals, and around 23 percent at Consumer Health.
Bayer has also prepared its guidance based on the closing exchange rates as of December 31, 2022, and the differences to the currency-adjusted forecast above are as follows: Group sales are expected to come in at 50 billion to 51 billion euros, and the Pharmaceuticals Division’s EBITDA margin before special items is projected to amount to around 30 percent.
Major strides in innovation and sustainability
Bayer has also made significant progress in launching and developing innovations. This is evident in the Pharmaceuticals Division, for instance, with the products Nubeqa™ and Kerendia™ as well as projects in late-stage development, such as asundexian for the prevention of stroke in atrial fibrillation patients and elinzanetant for the treatment of women in menopause. The company currently believes that these major growth drivers have a combined peak sales potential of over 12 billion euros annually. The Crop Science Division advanced the launch of new products that are designed to protect harvests even more effectively and reduce environmental impact. In the field of biologicals, it shifted its research approach to an open innovation model, with the division engaging in strategic collaborations with the Boston-based biotech company Ginkgo Bioworks and the Spanish biologicals company Kimitec, for example. Finally, the Consumer Health Division strengthened its product portfolio with the addition of Astepro™, the first steroid-free antihistamine spray on the US market that is available over the counter. It starts to work much faster, providing allergy sufferers with the relief they need.
Turning to the company’s sustainability targets, Baumann explained: “In 2022, we once again managed to reduce our greenhouse gas emissions overall – while at the same time achieving dynamic growth in our businesses.” The company’s efforts are gaining ever greater recognition, he remarked, with MSCI having upgraded its environmental, social and governance (ESG) rating for Bayer from “BB” to “A”. In addition, the company has for the first time made it into the top ten in the renowned Access to Medicine index, Baumann added. The index ranks companies in terms of their related endeavors in low- and middle-income countries. Bayer is also making good progress in attaining its ambitious social responsibility goals, he said. By 2030, the company aims to help 100 million smallholder farmers in low- and middle-income countries (LMICs), satisfy the need of 100 million women in LMICs for modern contraception, and support 100 million people in underserved communities with self-care.
Notes:
The following tables contain the key data for the Bayer Group and its divisions for the full year and the fourth quarter of 2022.
The complete Annual Report 2022 is available on the internet at:
www.bayer.com/annualreport
The Sustainability Report 2022 is also published on the internet at:
www.bayer.com/sustainability-report
The speech given by the Bayer Board of Management to the media will be available online from around 10 a.m. CET at:
www.bayer.com/speeches
Live broadcast of the Financial News Conference from around 10 a.m. CET and recording available from around 3 p.m. CET at:
www.bayer.com/live-mc
Additional information for investors, including presentation charts, and access to the live broadcast of the investor conference call (from around 2 p.m. CET) and recording (from around 6 p.m. CET) available at:
www.bayer.com/live-ic
Print-quality photos will be available
here
shortly after the news conference
Find more information at
www.bayer.com
.
Follow us on
twitter.com/bayer
Forward-Looking Statements
This release may contain forward-looking statements based on current assumptions and forecasts made by Bayer management. Various known and unknown risks, uncertainties and other factors could lead to material differences between the actual future results, financial situation, development or performance of the company and the estimates given here. These factors include those discussed in Bayer’s public reports which are available on the Bayer website at
www.bayer.com
. The company assumes no liability whatsoever to update these forward-looking statements or to conform
them to future events or developments.
Financial Statement
100 Deals associated with Iopromide
Login to view more data
R&D Status
10 top approved records. to view more data
Login
| Indication | Country/Location | Organization | Date |
|---|---|---|---|
| Breast Diseases | United States | 25 May 2023 | |
| Aortic Diseases | United States | 10 May 1995 | |
| Cardiovascular Diseases | United States | 10 May 1995 | |
| Cerebrovascular Disorders | United States | 10 May 1995 | |
| Coronary Disease | United States | 10 May 1995 | |
| Peripheral Arterial Disease | United States | 10 May 1995 | |
| Urethral Diseases | United States | 10 May 1995 | |
| Contrast agents | Australia | 12 Sep 1991 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Not Applicable | - | 132,850 | (Children (<18 years)) | bezbrowfwr(brzskjawsb) = rrawhthprb mihujmfdpt (ddqolgaeho ) | - | 01 Dec 2021 | |
(Elderly (≥65 years)) | bezbrowfwr(brzskjawsb) = ocoljolmgk mihujmfdpt (ddqolgaeho ) | ||||||
Phase 4 | - | 562 | gvvkzyoeaf(lezasfpdxv) = xzoyghpnoc uacdlcpmfl (qisrixmgqw ) View more | - | 22 Nov 2012 | ||
gvvkzyoeaf(lezasfpdxv) = heueskuvah uacdlcpmfl (qisrixmgqw ) View more | |||||||
Phase 3 | 435 | (Iopromide 370 mg I/mL) | yuzwvqqogv = ymgtwujaqa zlutxnxwyk (vqzqlghlpk, efikxrlxpe - fygyaeetop) View more | - | 20 Jul 2009 | ||
(Iopromide 300 mg I/mL) | yuzwvqqogv = darwgbtcwk zlutxnxwyk (vqzqlghlpk, ilxdioncwo - ycjdhjupaq) View more |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free


