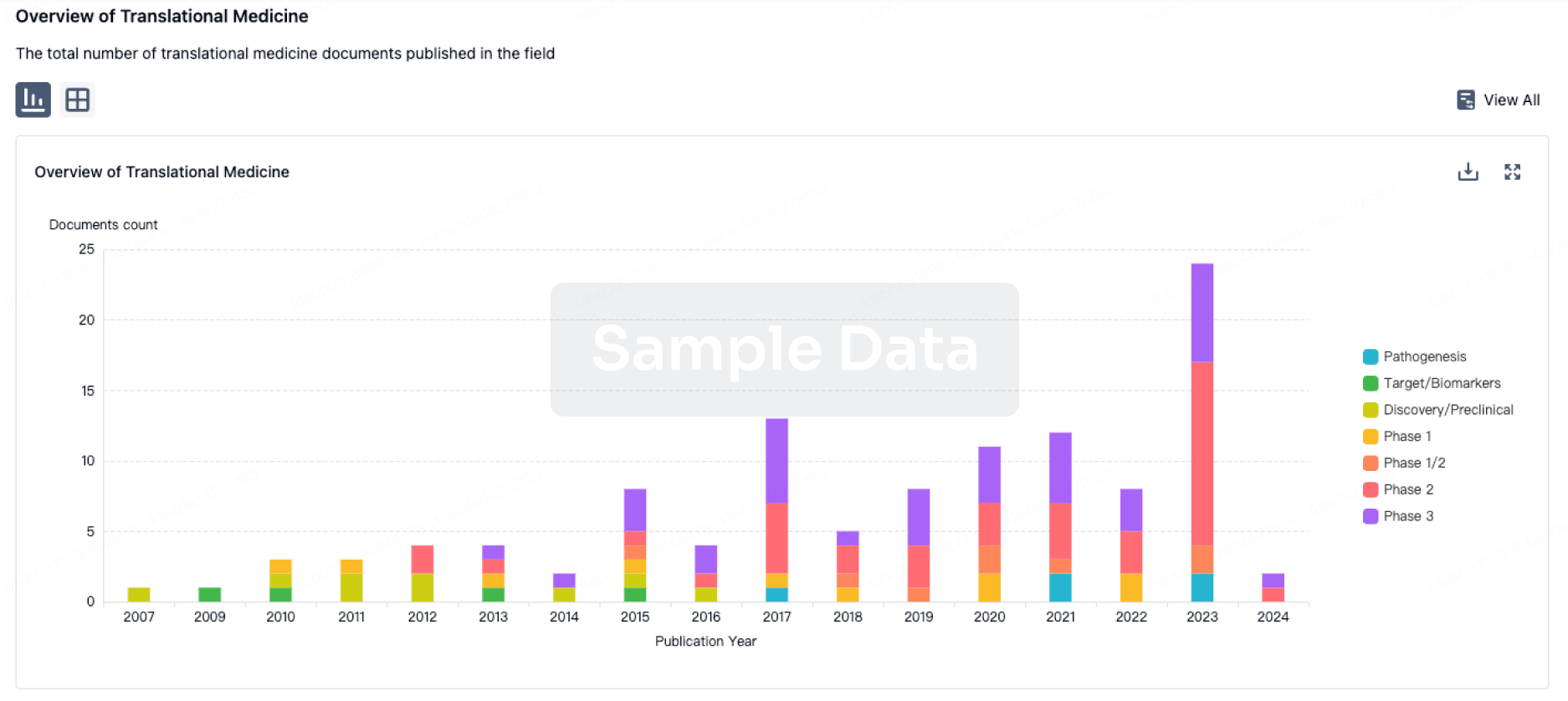
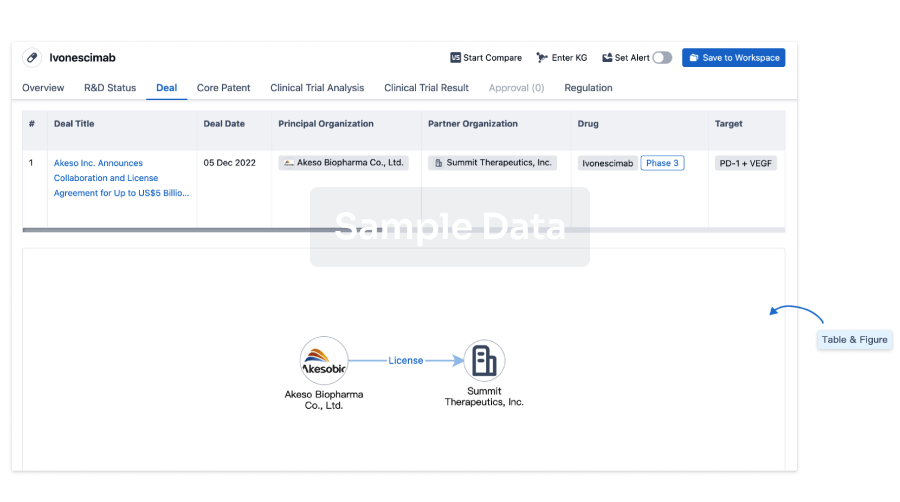
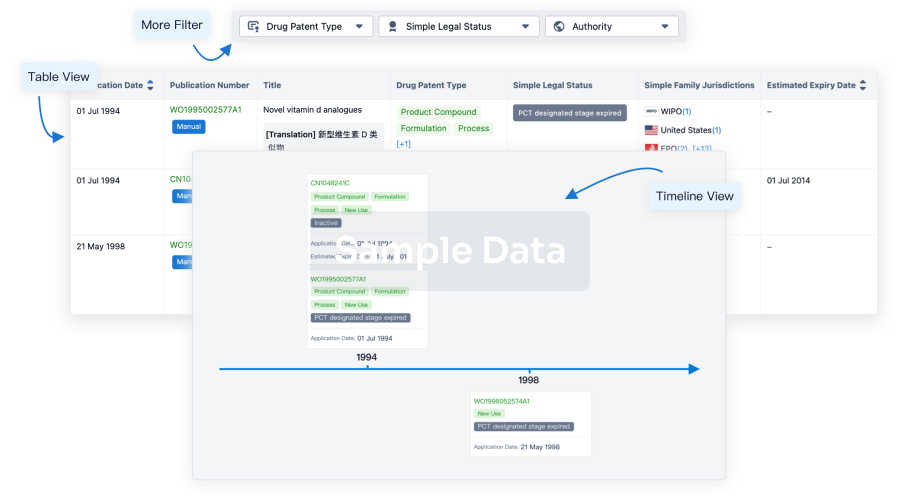
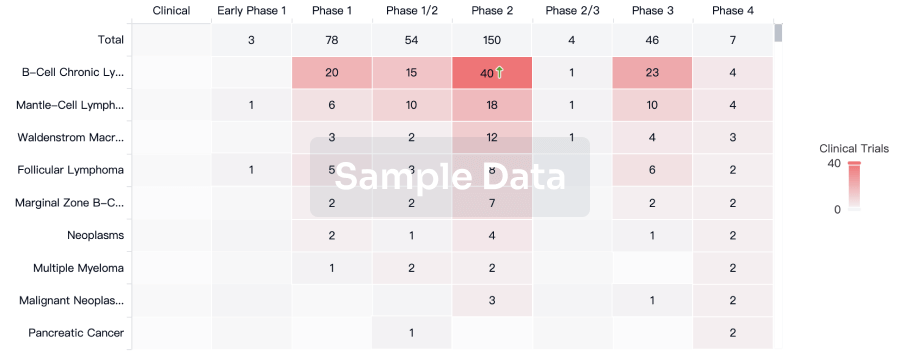
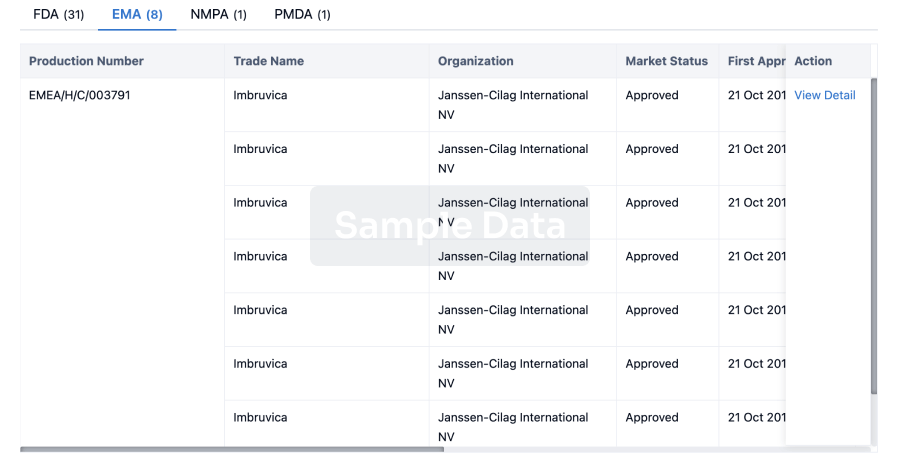
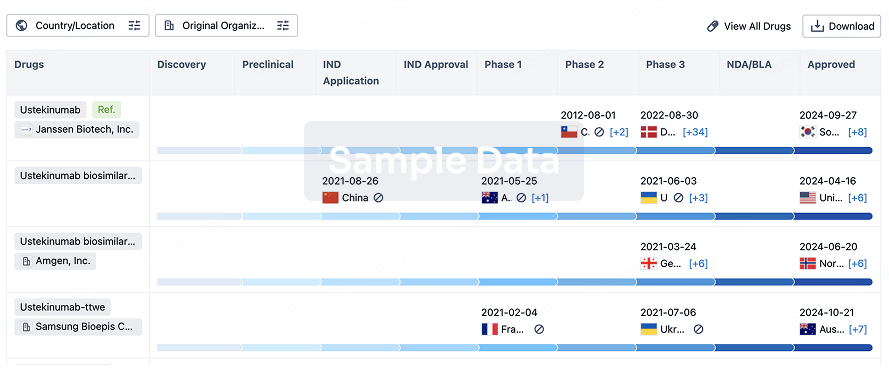
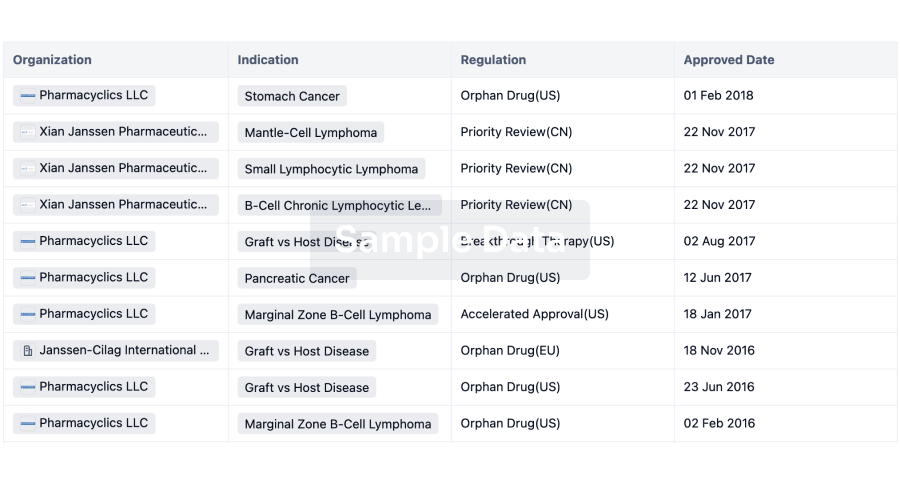
The prevalence of esophageal cancer has been rising since the past few years, which prompts the growing demand for the treatment options. The market is driven by the increasing prevalence of cancer and the increase in research & development. The Companies developing the potential therapies in the last stage of development include Celgene,Jiangsu HengRui Medicine Co., Seagen, and several others.
LAS VEGAS, Oct. 11, 2022 /PRNewswire/ -- DelveInsight's
'
Esophageal Cancer Pipeline Insight – 2022
' report provides comprehensive global coverage of available, marketed, and pipeline esophageal cancer therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the esophageal cancer pipeline domain.
Key Takeaways from the Esophageal Cancer Pipeline Report
DelveInsight's esophageal cancer pipeline report depicts a robust space with
80+ active players working to develop
90+ pipeline therapies for esophageal cancer treatment.
Leading esophageal cancer companies such as
Shanghai Henlius Biotech, CStone Pharmaceuticals, Jiangsu HengRui Medicine Co., Ltd., AstraZeneca, Symphogen, Hoffmann-La Roche, Chia Tai Tianqing Pharmaceutical Group Co., Ltd., Sinocelltech Ltd., Eli Lilly and Company, Ipsen, Jacobio Pharmaceuticals Co., Ltd., Shire, GlaxoSmithKline, Keythera Pharmaceuticals (Australia) Pty Ltd, Sunshine Lake Pharma Co., Ltd., Novartis Pharmaceuticals, Sanofi, Seagen Inc., Rapa Therapeutics LLC, Incyte Corporation, Atreca, Inc., Exelixis, Bayer, BeiGene, Lyell Immunopharma, Sichuan Baili Pharmaceutical, AP Biosciences.,Leap Therapeutics, Inc., Adlai Nortye Biopharma Co., Ltd., Athenex, Inc., Pfizer, Genentech, Hangzhou Neoantigen Therapeutics, Janssen Pharmaceutical, Curis, Inc.,Merck KGaA, Apexigen, Inc,Shenzhen Hornetcorn Bio-technology Company, LTD, MacroGenics, Bristol-Myers Squibb, Integral Molecular, CARTEXELL, EMD Serono, and others are evaluating new drugs for esophageal cancer treatment.
Key esophageal cancer pipeline therapies in various stages of development include
Serplulimab, CS1001, Camrelizumab, Durvalumab, Sym021, Spartalizumab, Sym022, Sym023, Regorafenib, S095033, INCB099318, RO7121661, XB002, LGK974, Abemaciclib, ladiratuzumab vedotin, ATRC-101, SGN-PDL1V, RO7247669, THOR-707, Letetresgene Autoleucel, TNO155, Anlotinib Hydrochloride, Larotinib, SCT-I10A, KF-0210, SCT200, Ramucirumab, Cabozantinib, JAB-3068, Onivyde, JAB-3312, SGN-B6A, RAPA-201,Ociperlimab, SI B001,Apatinib, AP 203, QEQ278, JAB-3312, DKN-01, AN0025, HR 070803,Oraxol, Sunitinib malate, Tiragolumab, iNeo Vac P01, Amivantamab, CA-4948, M1231, APX005M, DC-CIK, Margetuximab, Temozolomide, and others.
In
September 2022,
Daiichi Sankyo and
Sarah Cannon Research Institute (SCRI) announced that extended follow-up data from a phase I/II trial of
DS-7300, continued to show promising durable tumor response in patients with several types of heavily pretreated cancers including lung, prostate or esophageal cancer.A response rate of 32% (95% CI: 24-41) was observed with 38 responses (33 confirmed; 28%; 95% CI: 20-37) in 118 patients with various solid tumors including small cell lung cancer (SCLC), squamous non-small cell lung cancer (NSCLC), metastatic castration-resistant prostate cancer (CRPC), esophageal squamous cell carcinoma (ESCC), head and neck squamous cell carcinoma (HNSCC) or endometrial cancer receiving doses of DS-7300 ranging from 4.8 mg/kg to 16.0 mg/kg.
In
September 2022,
Integral Molecular, has
licensed a panel of monoclonal antibodies (MAbs) to
CARTEXELL, enabling CARTEXELL to develop CAR-T cell therapies using Integral Molecular's Claudin 18.2 (CLDN18.2) MAbs. Under the terms of the agreement, Integral Molecular will provide an exclusive worldwide license to CARTEXELL to use the panel of high-affinity, high-specificity, and fully humanized CLND18.2 MAbs for the development of CAR-T cell therapies against solid tumors including gastric, lung, pancreatic and esophageal cancers. CARTEXELL will be solely responsible for all research, development, and commercial activities.
In
August 2022,
BeiGene announced that the
Center for Drug Evaluation (CDE) of the China
National Medical Products Administration (NMPA) has accepted a
supplemental biologics license application (sBLA) for
tislelizumab in combination with chemotherapy as first-line treatment in patients with unresectable locally advanced, recurrent or metastatic esophageal squamous cell carcinoma (ESCC).
In
May 2022,
Shanghai Junshi Biosciences Co., Ltd, announced that the
China National Medical Products Administration (NMPA) has approved the
supplemental new drug application (sNDA) for
toripalimab in combination with paclitaxel and cisplatin in the first-line treatment of patients with unresectable locally advanced/recurrent or distant metastatic esophageal squamous cell carcinoma (ESCC). The sNDA was accepted by the NMPA in July 2021. This is the fifth indication approved for toripalimab in China and will benefit Chinese patients with advanced ESCC.
In
May 2022,
Bristol Myers Squibb, announced that the U.S.
Food and Drug Administration (FDA) has
approved both
Opdivo (nivolumab) (injection for intravenous use) in combination with fluoropyrimidine- and platinum-containing chemotherapy and
Opdivo plus Yervoy (ipilimumab) as a first-line treatment for adult patients with unresectable advanced or metastatic esophageal squamous cell carcinoma (ESCC) regardless of PD-L1 status. The approvals are based on the Phase III CheckMate -648 trial, which evaluated Opdivo in combination with chemotherapy (n=321) and Opdivo plus Yervoy (n=325) each compared to chemotherapy alone (n=324), and was the largest Phase III trial of immunotherapy in first-line ESCC.
In
January 2022,
Lyell Immunopharma, Inc., announced that the U.S.
Food and Drug Administration (FDA) has cleared an
Investigational New Drug (IND) application to initiate a Phase I clinical trial for
LYL132, an investigational T-cell receptor (TCR) therapy for patients with solid tumors expressing New York esophageal squamous cell carcinoma 1 (NY-ESO-1) that the company is developing in collaboration with GSK.
Request a sample and discover the recent advances in esophageal cancer treatment @
Esophageal Cancer Pipeline Report
The esophageal cancer pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage esophageal cancer drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the esophageal cancer pipeline landscape.
Esophageal Cancer Overview
Esophageal cancer grows in the esophagus, the tube that connects the throat to the stomach. Tumors develop in the mucosa, the inner lining of the esophagus. Esophageal cancer is classified into two types, each with its own set of risk factors: Adenocarcinoma -Adenocarcinomas are cancers that begin in gland cells at the bottom of the esophagus. This is the most common type of esophageal cancer. It usually happens near the stomach. The exact esophageal cancer causes are not known.
Most esophageal cancer symptoms do not appear until cancer has progressed to an advanced stage, when it may be challenging to treat. Other, more common conditions can cause esophageal cancer symptoms. The most common esophageal cancer symptom is difficulty swallowing, particularly a sensation of food stuck in the chest.
There are several tests used for esophageal cancer diagnosis. The most common include endoscopy with biopsy and endoscopic ultrasonography.
Find out more about esophageal cancer drugs in development @
New Treatment for Esophageal Cancer
A snapshot of the Esophageal Cancer Pipeline Drugs mentioned in the report:
Learn more about the emerging esophageal cancer pipeline therapies @
Esophageal Cancer Clinical Trials
Esophageal Cancer Therapeutics Assessment
The
esophageal cancer pipeline report proffers an integral view of esophageal cancer emerging novel therapies segmented by stage, product type, molecule type, mechanism of action, and route of administration.
Scope of the Esophageal Cancer Pipeline Report
Coverage: Global
Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
Therapeutics Assessment
By Route of Administration: Intra-articular, Intraocular, Intrathecal, Intravenous, Ophthalmic, Oral, Parenteral, Subcutaneous, Topical, Transdermal
Therapeutics Assessment
By Molecule Type: Oligonucleotide, Peptide, Small molecule
Therapeutics Assessment
By Mechanism of Action: Prostaglandin E EP4 receptor antagonists, ERBB 2 receptor antagonists, Phosphorylation inhibitors, Antibody-dependent cell cytotoxicity, Apoptosis stimulants, CD40 antigen stimulants, Immunostimulants, Programmed cell death-1 receptor antagonists, T lymphocyte stimulants, Programmed cell death-1 ligand-1 inhibitors, Epidermal growth factor receptor antagonists.
Key Esophageal Cancer Companies: Shanghai Henlius Biotech, CStone Pharmaceuticals, Jiangsu HengRui Medicine Co., Ltd., AstraZeneca, Symphogen, Hoffmann-La Roche, Chia Tai Tianqing Pharmaceutical Group Co., Ltd., Sinocelltech Ltd., Eli Lilly and Company, Ipsen, Jacobio Pharmaceuticals Co., Ltd., Shire, GlaxoSmithKline, Keythera Pharmaceuticals (Australia) Pty Ltd, Sunshine Lake Pharma Co., Ltd., Novartis Pharmaceuticals, Sanofi, Seagen Inc., Rapa Therapeutics LLC, Incyte Corporation, Atreca, Inc., Exelixis, Bayer, BeiGene, Lyell Immunopharma, Sichuan Baili Pharmaceutical, AP Biosciences.,Leap Therapeutics, Inc., Adlai Nortye Biopharma Co., Ltd., Athenex, Inc., Pfizer, Genentech, Hangzhou Neoantigen Therapeutics, Janssen Pharmaceutical, Curis, Inc.,Merck KGaA, Apexigen, Inc,Shenzhen Hornetcorn Bio-technology Company, LTD, MacroGenics, Bristol-Myers Squibb, Integral Molecular, CARTEXELL and others
Key Esophageal Cancer Pipeline Therapies: Serplulimab, CS1001, Camrelizumab, Durvalumab, Sym021, Spartalizumab, Sym022, Sym023, Regorafenib, S095033, INCB099318, RO7121661, XB002, LGK974, Abemaciclib, ladiratuzumab vedotin, ATRC-101, SGN-PDL1V, RO7247669, THOR-707, Letetresgene Autoleucel, TNO155, Anlotinib Hydrochloride, Larotinib, SCT-I10A, KF-0210, SCT200, Ramucirumab, Cabozantinib, JAB-3068, Onivyde, JAB-3312, SGN-B6A, RAPA-201,Ociperlimab, SI B001,Apatinib, AP 203, QEQ278, JAB-3312, DKN-01, AN0025, HR 070803,Oraxol, Sunitinib malate, Tiragolumab, iNeo Vac P01, Amivantamab, CA-4948, M1231, APX005M, DC-CIK, Margetuximab, Temozolomide and others.
Dive deep into rich insights for esophageal cancer drugs, visit @
Chemo Drugs for Esophageal Cancer Treatment
Table of Contents
For further information on the esophageal cancer pipeline therapeutics, reach out @
New Drug for Esophageal Cancer Treatment
Related Reports
Esophageal Cancer Market
Esophageal Cancer Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key esophageal cancer companies including
Merck KGaA, Shenzhen Hornetcorn Biotechnology, Apexigen, Inc., Highlight Therapeutics, among others.
Esophageal Cancer Epidemiology Forecast
Esophageal Cancer Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted esophageal cancer epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Esophageal Squamous Cell Carcinoma Pipeline
Esophageal Squamous Cell Carcinoma Pipeline Insight – 2022 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key esophageal squamous cell carcinoma companies, including
BeiGene, AstraZeneca, Ipsen, Shire, Sanofi, Seagen, among others.
Esophageal Squamous Carcinoma Market
Esophageal Squamous Carcinoma Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key esophageal squamous carcinoma companies, including
BeiGene, AstraZeneca, Ipsen, Shire, Sanofi, Seagen, among others.
Esophageal Squamous Cell Carcinoma Epidemiology
Esophageal Squamous Cell Carcinoma Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted esophageal squamous cell carcinoma epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Other Trending Reports
Goitre Market
|
Thymus Cancer Market
|
US Healthcare Outlook Report
|
Venous Stenosis Market
|
Negative Pressure Wound Therapy Systems Market
|
Global Kinase Inhibitor in Autoimmune Diseases Market
|
Metrorrhagia Market
|
Dysfunctional Uterine Bleeding Market
|
Hypereosinophilic Syndrome Market
|
Age-related Vision Dysfunction Market
|
Dental Lasers Market
|
CRISPR Therapies Pipeline Insight
|
Cell And Gene Therapy For Multiple Myeloma Market
|
Drug Hypersensitivity Market
|
Dysthymia Market
|
Persistent Depressive Disorder Market
|
Cancer Vaccines Market
|
Weight Loss/Weight Management (Obesity) Market |
Food Allergy Market |
Obsessive-Compulsive Disorder Market |
Tumor Ablation Market |
Physiotherapy Equipment Market |
Chimeric Antigen Receptor (CAR) T-Cell Therapy Market |
Anti-hypertension Market |
Radiofrequency Ablation Devices Market |
Global Messenger RNA (mRNA)-based Vaccines and Therapeutics Market |
Gastro Intestinal Bleeding Market |
Trastuzumab Biosimilars Insight |
Varicose Veins Market |
Germ Cell Tumor Market |
Intracardiac Echocardiography Devices Market |
India Healthcare Report |
Crows Feet Market |
Seborrhoeic Dermatitis Market |
Injectable Drug Delivery Devices Market |
Structural Heart Devices Market |
Substance (Drug) Abuse Market |
Insulin Glargine Biosimilar Insight
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn
Contact Us
Shruti Thakur
[email protected]
+1(919)321-6187
Logo:
SOURCE DelveInsight Business Research, LLP