Request Demo
Last update 06 Dec 2025
Revusiran
Last update 06 Dec 2025
Overview
Basic Info
Drug Type siRNA |
Synonyms ALN-TTRsc, siTTRsc, AD-51547 + [2] |
Target |
Action inhibitors |
Mechanism TTR inhibitors(Transthyretin inhibitors), RNA interference |
Therapeutic Areas |
Active Indication- |
Originator Organization |
Active Organization- |
Inactive Organization |
License Organization |
Drug Highest PhaseDiscontinuedPhase 3 |
First Approval Date- |
RegulationOrphan Drug (United States) |
Login to view timeline
Structure/Sequence
Boost your research with our RNA technology data.
login
or
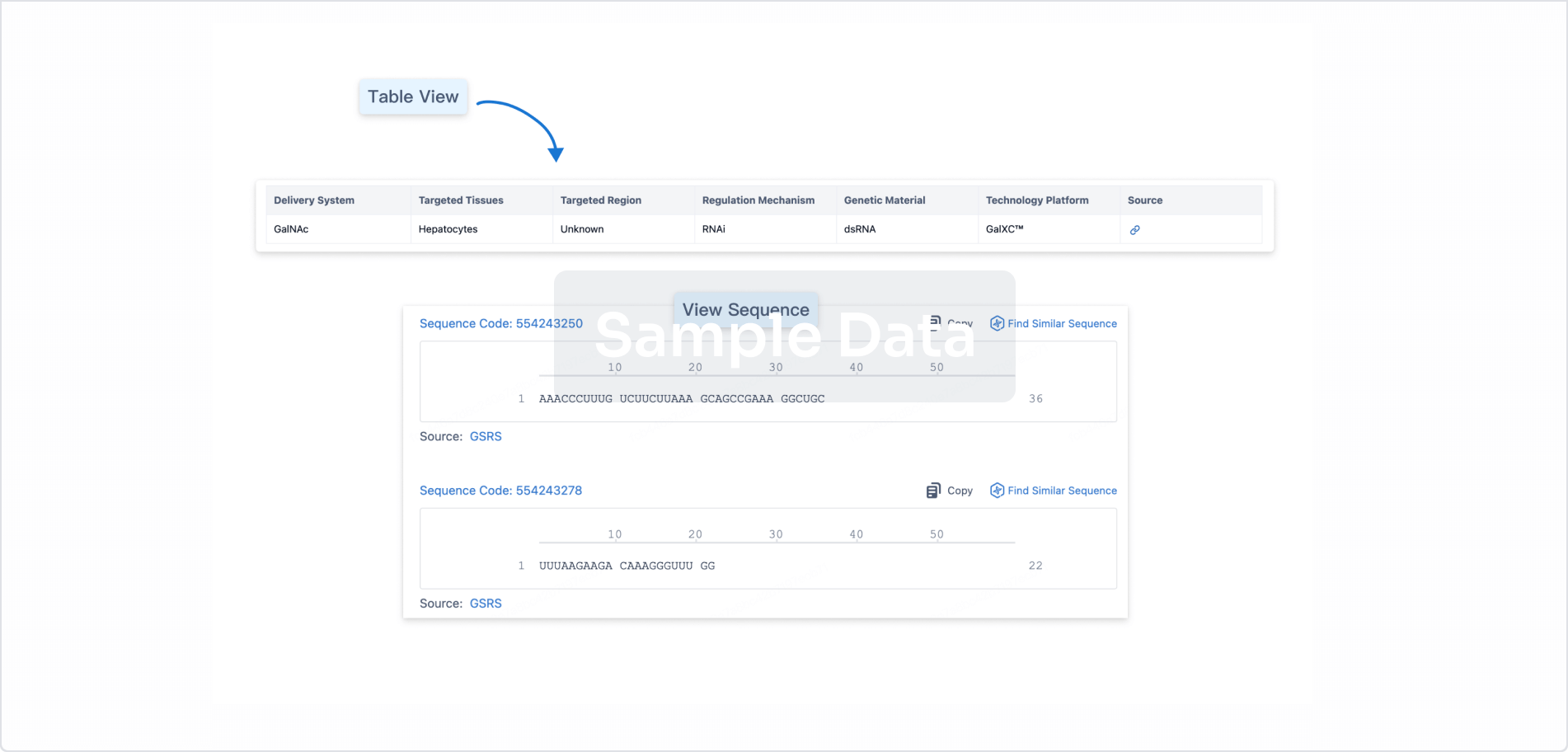
Sequence Code 30033233

Source: *****
Sequence Code 719622178

Source: *****
Related
6
Clinical Trials associated with RevusiranEUCTR2015-005333-49-GB
A Multicenter, Multinational, Open-label Extension Study to Evaluate the Long-term Safety and Efficacy of Revusiran in Patients with Transthyretin-mediated Familial Amyloidotic Cardiomyopathy
Start Date30 Jun 2016 |
Sponsor / Collaborator |
NCT02595983
An Open-label Study to Evaluate the Efficacy and Safety of Revusiran in Patients With Transthyretin-mediated Familial Amyloidotic Polyneuropathy With Disease Progression Post-Orthotopic Liver Transplant
The purpose of this study was to evaluate the safety and effectiveness of revusiran (ALN-TTRSC) in adults with transthyretin-mediated amyloidosis (ATTR), whose disease has continued to worsen after liver transplantation. Dosing has been discontinued; patients are being followed-up for safety.
Start Date01 Oct 2015 |
Sponsor / Collaborator |
NCT02319005
A Phase 3 Multicenter, Multinational, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of ALN TTRSC in Patients With Transthyretin (TTR) Mediated Familial Amyloidotic Cardiomyopathy (FAC)
The purpose of this study was to evaluate the safety and efficacy of revusiran (ALN-TTRSC) in patients with transthyretin (TTR) mediated Familial Amyloidotic Cardiomyopathy. Dosing has been discontinued; patients are being followed-up for safety.
Start Date01 Dec 2014 |
Sponsor / Collaborator |
100 Clinical Results associated with Revusiran
Login to view more data
100 Translational Medicine associated with Revusiran
Login to view more data
100 Patents (Medical) associated with Revusiran
Login to view more data
12
Literatures (Medical) associated with Revusiran01 Nov 2024·HEART & LUNG
Safety and effectiveness of interference RNA (RNAi) based therapeutics in cardiac failure: A systematic review
Review
Author: Iqbal, Javed ; Bashir, Muhammad Atif ; Farhan, Eesha ; Shahid, Fatima ; Qadri, Maria ; Jain, Hritvik ; Saddique, Muhammad Nabeel ; Ain, Noor Ul ; Benyamin, Javeria
BACKGROUND:
Heart failure is a major worldwide health concern and leading cause of mortality. RNAi interventions hold promise for patients resistant to conventional drugs due to their off-target effects and lack of specificity.
OBJECTIVES:
To examine the safety and effectiveness of RNAi therapeutics in treating heart failure.
METHODS:
The PubMed, Embase, Scopus and Cochrane databases were searched using appropriate keyword from inception until December 31, 2023. A total of 14 studies fulfilling predefined selection criteria were included for qualitative synthesis.
RESULTS:
We found that in patients with cardiac amyloidosis, patisiran and revusiran showed considerable improvements in cardiac output and left ventricular wall thickness. In animal studies, Nox2-siRNA showed effectiveness in regaining heart function. Furthermore, cardiomyocyte count and left ventricular function were improved by DUSP5 siRNA + T3 therapy and meg3 inhibition after myocardial infarction (MI). RNAi showed minimal adverse effects like peripheral neuropathy, hepatotoxicity, urinary tract infection, vaginal infection, diarrhea, abdominal pain arrhythmias, conduction disorders, and cardiotoxicity (LV wall thinning, heart failure) and improved cardiac biomarkers.
CONCLUSION:
RNAi therapeutics are novel treatment option for improving cardiac function because their high target specificity, ability to target genes that conventional drugs struggle to reach and potential for long-lasting effects. Further research on optimizing delivery methods, improving target specificity, evaluating long-term safety profiles and cost-effectiveness to fully realize their potential.
04 Oct 2024·European Heart Journal-Cardiovascular Pharmacotherapy
Pharmacological management of transthyretin amyloid cardiomyopathy: a scoping review
Article
Author: Ibrahim, Ramzi ; Raza, Shahzad ; Asif, Mohammad Shahzad ; Ahmad, Fahad ; Masthan, Shameera Shaik ; Pham, Hoang Nhat ; Anwar, Zain ; Safdar, Ahmad ; Hassan, Danial ; Rehman, Shafi ; William, Preethi
Abstract:
Aims:
Transthyretin amyloid cardiomyopathy (ATTR-CM) is characterized by the accumulation of transthyretin (TTR) protein in the myocardium. The aim of this scoping review is to provide a descriptive summary of the clinical trials and observational studies that evaluated the clinical efficacy and safety of various agents used in ATTR-CM, with a goal of identifying the contemporary gaps in literature and to reveal future research opportunities.
Methods and results:
The search was performed in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A literature search using several databases for observational and clinical trials investigating the treatment modalities for ATTR-CM was undertaken. We extracted data including study characteristics, primary endpoints, and adverse events from each study. A total of 19 studies were included in our scoping review. Out of which, 8 were clinical trials and 11 were observational analyses. The drugs evaluated included tafamadis, acoramidis, revusiran, doxycycline and tauroursodeoxycholic acid and doxycycline, diflusinil, inotersan, eplontersen, and patisiran. Tafamidis has shown to be efficacious in the management of ATTR-CM, particularly when initiated at earlier stages. RNA interference and antisense oligonucleotide drugs have shown promising impacts on quality of life. Additionally, this review identified gaps in the literature, particularly among long-term outcomes, comparative effectiveness, and the translation of research into economic contexts.
Conclusion:
Multiple pharmacological options are potential disease-modifying therapies for ATTR-CM. However, many gaps exist in the understanding of these various drug therapies, warranting further research. The future directions for management of ATTR-CM are promising in regard to improving prognostic implications.
11 Jan 2021·Journal of neuromuscular diseases
Targeted Therapies for Hereditary Peripheral Neuropathies: Systematic Review and Steps Towards a ‘treatabolome’
Review
Author: Lochmüller, Angela ; Atalaia, Antonio ; Horvath, Rita ; Jennings, Matthew J.
Background::
Hereditary peripheral neuropathies are inherited disorders affecting the peripheral nervous system, including Charcot-Marie-Tooth disease, familial amyloid polyneuropathy and hereditary sensory and motor neuropathies. While the molecular basis of hereditary peripheral neuropathies has been extensively researched, interventional trials of pharmacological therapies are lacking.
Objective::
We collated evidence for the effectiveness of pharmacological and gene-based treatments for hereditary peripheral neuropathies.
Methods::
We searched several databases for randomised controlled trials (RCT), observational studies and case reports of therapies in hereditary peripheral neuropathies. Two investigators extracted and analysed the data independently, assessing study quality using the Oxford Centre for Evidence Based Medicine 2011 Levels of Evidence in conjunction with the Jadad scale.
Results::
Of the 2046 studies initially identified, 119 trials met our inclusion criteria, of which only 34 were carried over into our final analysis. Ascorbic acid was shown to have no therapeutic benefit in CMT1A, while a combination of baclofen, naltrexone and sorbitol (PXT3003) demonstrated some efficacy, but phase III data are incomplete. In TTR-related amyloid polyneuropathy tafamidis, patisiran, inotersen and revusiran showed significant benefit in high quality RCTs. Smaller studies showed the efficacy of L-serine for SPTLC1-related hereditary sensory neuropathy, riboflavin for Brown-Vialetto-Van Laere syndrome ( SLC52A2/3) and phytanic acid-poor diet in Refsum disease ( PHYH).
Conclusions::
The ‘treatable’ variants highlighted in this project will be flagged in the treatabolome database to alert clinicians at the time of the diagnosis and enable timely treatment of patients with hereditary peripheral neuropathies.
10
News (Medical) associated with Revusiran20 Sep 2017
September 20, 2017
By
Alex Keown
, BioSpace.com Breaking News Staff
CAMBRIDGE, Mass. –
Positive Phase III data
for
Alnylam
’s RNA interference treatment for hereditary ATTR amyloidosis with polyneuropathy has caused shares of company stock to spike more than 23 percent in premarket trading.
This morning, the company announced its Phase III Apollo study for patisiran met its primary efficacy endpoint and all secondary endpoints. Alnylam said patients on the patisiran arm fared significantly better against placebo. At 18 months, the mean change from baseline in mNIS+7 was significantly lower in the patisiran group as compared with placebo, Alnylam said. Trial data shows mean and median changes in mNIS+7 impairment scores for patisiran patients were both negative values. That indicates an overall improvement in the majority of patients compared with baseline, Alnylam said.
Based on the positive results, Alnylam and its developmental partner
Sanofi
plan to regulatory approval by the end of the year.
Sanofi Genzyme
is currently preparing for regulatory filings for patisiran in Japan, Brazil and other countries, to begin in the first half of 2018. Investors and company leaders are breathing a sigh of relief as Alnylam’s long journey to develop RNAi treatments appears to be paying off.
Alnylam Chief Executive Officer
John Maraganore
said the positive data is the “culmination of a 15-year journey of tireless work.” Now RNAi therapeutics are a reality, he said. RNAi is a natural mechanism of gene silencing.
“This is an incredibly exciting milestone for Alnylam and RNAi, and most importantly for patients and their treating physicians and families. We extend our deepest gratitude to all the patients, investigators and study staff who participated in the Apollo study – they made this important scientific progress possible,”
Maraganore
said in a statement this morning.
TTR amyloidosis is a severe, progressive and fatal disease with multiple overlapping clinical manifestations. There are three forms of TTR amyloidosis: familial amyloid polyneuropathy, FAP, familial amyloid cardiomyopathy, FAC, and wild type (wt)-TTR amyloidosis. The disease is caused by the accumulation of misfolded TTR protein in a broad range of tissues and organs, including peripheral nerves, heart, intestinal tract, eyes, kidneys, central nervous system, thyroid and bone. The progressive accumulation of TTR amyloid deposits in these tissues and organs leads to organ failure and eventually death.
Elias Zerhouni
, head of Sanofi’s global R&D team, added that the results from the Apollo trial “supports our belief that RNAi therapeutics have the potential to become an innovative new class of medicines for patients with rare genetic diseases.” He said trial data indicates that patisiran could help “improve the lives of people living with hATTR amyloidosis with polyneuropathy, a patient population in urgent need of additional treatment options.”
Alnylam’s RNAi therapies have gone through a wild ride over the years of development. Last year, Alnylam announced it was
discontinuing development of revusiran
for hereditary ATTR amyloidosis with cardiomyopathy (hATTR-CM) following patient deaths.
As Alnylam investors celebrate the positive data this morning, investors in rival RNAi company
Ionis Pharmaceuticals
are not. Shares of that company are down about 10 percent in early trading. Ionis has
seen several setbacks with its RNAi therapy
targeting TTR amyloid cardiomyopathy, which includes patients with familial amyloid cardiomyopathy (FAC) and patients with wild-type transthyretin amyloidosis (wt-TTR amyloidosis).
While Alnylam celebrates the Apollo trial, the company saw a setback earlier this month when it
suspended development
of its Phase II fitusiran trial for hemophilia A following the death of a patient. The patient died from a thrombotic event and the company said it was suspending the trial pending further investigation.
Phase 3Phase 2Clinical ResultsiRNA
07 Dec 2016
December 14, 2016
By
Karl Thiel
for BioSpace.com
Many of us will be glad to see the end of 2016, a year that was marked by terrorism both domestic and international, a string of celebrity deaths, a new disease scourge (Zika became the new Ebola), and a whole lot of political turmoil that made for some tense Thanksgiving table conversations.
Here in our little corner of the world, there was plenty of volatility too. So without further delay, let’s count down this year’s wholly subjective list of the year’s top 10 biotech trends and events.
#10: Brexit.
The U.K.'s decision to exit the European Union will likely have some broad-reaching and long-lasting effects, most of which are yet to be felt. In the short term, the move has been on balance positive for U.K.'s pharma sector—for a simple reason. A historically weak pound sterling is helping companies like
GlaxoSmithKline
and
AstraZeneca
boost dollar-denominated profits.
Rumors initially swirled that both these pharma giants would pull up roots and leave the U.K., but that doesn’t appear to be the case, at least for now. Longer term, it remains to be seen if some of the bigger fears—failure to attract or retain top talent, securing investment, and keeping favorable trade deals—come to fruition.
#9: Sharply declining approvals.
Both 2014 and 2015 were tough acts to follow. There were 45 novel drugs approved last year; 41 the year before. As I write, there have only been 19 novel drugs approved in 2016—putting this on track to be the worst year since 2007. This isn’t necessarily the
FDA’s
fault—they can only approve the applications these receive…and some would argue that the agency has, if anything, been overly lenient in granting approvals this year.
#8: Lots of big clinical failures.
While 2016 has certainly had its bright spots, the year has been more notable for high-pro failures than for its successes. There’s
BioMarin’s
drisapersen,
Celldex Therapeutics’
Rintega,
Eli Lilly’s
solenuzumab,
Alnylam’s
Revusiran,
Clovis Oncology’s
rociletinib, and
Bristol Myers-Squibb’s
Opdivo in lung cancer.
Juno Therapeutics
has run into some serious, perhaps program-ending difficulties with JCAR-015.
Gilead
has had a bad run of luck with almost everything it touched this year, from GS-5745 (in ulcerative colitis) to simtuzumab to eleclazine to Zydelig (in first-line cancer applications).
Bluebird Bio
went from hot to not after turning out disappointing early results in its sickle cell programs.
There have been some great achievements (see below)—but it’s hard to counter-balance this depressing list.
#7: The drug price debate continues, then stops, then starts up again.
Drug companies always make juicy political targets, so it’s no surprise that the heat stayed high all during this election year. It’s been a debate long on rhetoric and short on substance, with the main result being that companies grew more eager to get in price increases before the music stopped. Some, of course, were a little more ham-fisted about than others (cough,
Mylan
).
Pharma investors breathed a sigh of relief after the election of
Donald Trump
(see point #1 below), but that sense of resolution may have come too early.
Also, don’t forget about some of the most visible faces of price-gouging—
Valeant
subsidiary
Philidor
and its CEO have had criminal charges filed against them, and there may be more to come.
Martin Shkreli
is guaranteed to be back in the news in 2017, since that’s when his criminal fraud trial is likely to take place.
#6: M&A slowed to a comparative crawl.
2015 was a record-breaking year for biopharma M&A, with some $330 billion in deals, even after you factor out the $183 billion
Pfizer
-
Allergan
link-up that never happened. Few expected this year to match that sum, but fewer still expected the totals to drop so sharply. The final tallies won’t be in until the New Year, but 2016 was recently running under $100 billion in pharma and biotech M&A.
Will a flood of repatriated cash, courtesy of a new Trump tax policy, change the calculus in 2017?
I have my doubts
(and not only because it will take a while to change the tax code, even with a willing Congress). But it wouldn’t be surprising to see the pace pick up at least a little next year.
#5: Market meltdown.
The “Trump bump” to the stock market—and the biotech market in particular—was nice for investors while it lasted. Even at its best, it did little to erase what happened in the opening weeks of 2016. As we stand now, the Nasdaq Biotech Index, up less than 2 percent since the election. It is down over 20 percent year-to-date. A number of other points in this list help explain why.
#4: The (controversial) rise of antisense.
Antisense has been kicking around since the 1980s without much commercial success. While that technically continued to be true this year, it’s about to change. Spinraza, the antisense drug for spinal muscular atrophy developed by
Ionis Pharma
and
Biogen
, produced strong enough results in a study of infantile onset spinal muscular atrophy that the trial was stopped early. Since then, robust signals have been seen in patients with later-onset disease. And the drug reportedly has a reasonably clean safety pro since side effects and safety concerns have dogged some other antisense drugs.
Then there’s
Sarepta’s
eteplirsen, trade-named Exondys 51, approved to treat Duchenne muscular dystrophy is a specific subset of patients. The decision to approve was controversial and, according to some,
undermined the credibility
of the FDA. Certainly it remains unclear whether the drug’s miniscule effect on dystrophin production will create a meaningful change in the course of the disease. But that probably won’t stop it from racking up hundreds of millions of dollars in sales.
#3: CAR-T meltdown?
Cancer immunotherapy has been so hot over the past few years that some pundits have bemoaned a lack of research dollars going elsewhere. CAR-T seems like an obvious next step—a way to program T-cells to attack specific cancers. And early clinical results have shown astounding success, producing apparent cures in leukemia. But the therapy has turned out to be quite dangerous in some situations, dousing another fire that burned so bright for biotech in 2015.
That’s been particularly true for
Juno Therapeutics
, which has put its pivotal trial of JCAR-015 on clinical hold twice.
Novartis
, once arguably the leader in the area, seemingly stepped back from CAR-T this past summer, dissolving its dedicated unit, bringing programs back into the larger organization and perhaps putting less emphasis on the technology (though they continue to push forward).
Kite Pharma
is the beneficiary of these developments, yet investors are regarding the company warily. The stock is down sharply for the year and well off its 52-week high even as it files for its first approval.
Call it another case of hype waking up to reality: CAR-T remains a very promising technology, but it’s still unclear how effective it will be outside of blood cancers, and how it will be used even in leukemias, where oncologists will have to weigh up robust results against serious potential risks.
#2: CRISPR goes public.
At the start of the year, there were zero pure-play companies in the CRISPR space. Now there are three—
Editas Medicine
,
Intellia Therapeutics
, and
CRISPR Therapeutics
—not to mention a bunch more companies dabbling in the space. But these companies—which have had a mixed-at-best reception with investors—are only the tip of the iceberg, held back in part by complex and acrimonious intellectual property disputes.
CRISPR has exploded around the world, with the
University of Pennsylvania
planning a seeming Hail Mary pass into clinical trials thanks to funding from the Parker Institute, only to be beaten to the punch by
Sichuan University
in China.
Even without clinical trials, the technology is making all sorts of experiments possible—even if some raise safety or ethical issues. By this time next year, perhaps we’ll all have access to
giant CRISPR beagles
.
#1: Trump elected president.
This election upset takes the number one spot. But what it means for the biotech sector (or anything else) remains unclear. Trump has certainly been known to spout anti-science rhetoric and anti-vaxxer drivel. He’s said he wants to cut drug prices, using mechanisms like reimportation and direct Medicare negotiation. But then he went mum on those topics. And then he started up again, so who knows? He has said he wants to streamline FDA and get medicines to people more quickly, pushing a popular political hot button. But does that mean a bigger FDA budget, laxer standards, or something else?
It seems likely, based on both his rhetoric and his choice of Rep.
Tom Price
(R-Ga.) as
Secretary of Health and Human Services
, that he plans to make good on his promise to repeal and replace Obamacare. Yet how will that play out? The Affordable Care Act has generally been popular with drug companies, because it has given them millions of new customers. But it has also come with new regulatory burdens, like requirements to compile and submit data to Patient Centered Outcomes Research Institutes. What is simply repealed and what is replaced will have a big impact on companies.
Here’s another irony: One interesting impact of Obamacare is that it gives the president fairly broad authority to enact price controls when Medicare grows at a certain level beyond inflation—as it is expected to do in 2017 for the first time since the law was passed. So biopharma companies who’ve thus far seen the most favorable side of the law may be saved from its restrictions just before the hammer comes down—if Trump and Congress do indeed repeal. But maybe Trump will decide he likes the authority in the current law.
However it goes down, it’s going to be an interesting 2017.
-
Karl Thiel
Read the
BioPharm Executive
online newsletter December 14, 2016.
Sign-up for the
free monthly subscription
to the
BioPharm Executive
Related Jobs
VP, Clinical Development
Director, Genomics
VP, Clinical Development
Director of Operations
VP, Sales
Director, Biostatistics
View More Jobs
Drug ApprovalImmunotherapy
04 Nov 2016
November 4, 2016
By
Mark Terry
, BioSpace.com Breaking News Staff
On October 5, Cambridge, Mass.-based
Alnylam Pharmaceuticals
announced
that it was discontinuing development of revusiran for hereditary ATTR amyloidosis with cardiomyopathy (hATTR-CM). The decision was made on the basis of unexpected patient deaths. At its
third-quarter conference call
the company provided more details.
Alnylam works in the field of RNA interference (RNAi). This is based on how genes are turned on and off. ATTR amyloidosis is a progressively debilitating and often fatal disease caused by an accumulation of transthyretin (TTR) in peripheral tissues. The TTR protein is mostly produced in the liver and normally carries vitamin A.
On October 5, the company disclosed that there had been 18 patient deaths in the trial. During the conference call, the company’s chief medical officer,
Akshay Vaishnaw
, said that there had been another death. Of the original 18 deaths, 16 were receiving revusiran.
The trial has 206 patients, all with ATTR cardiomyopathy. They were randomized two-to-one to receive revusiran versus placebo. Vaishnaw said there was a “perfectly balanced distribution” of the deaths between the active drug arm and the placebo arm.
Although the data monitoring committee recommended the trial be halted, the company has said “there was no conclusive evidence of drug-related peripheral neuropathy” that would have caused the deaths.
“We have established a team to evaluate all the revusiran data,” Vaishnaw said during the call. “To the extent possible, we intend to provide you with an update on our progress at our upcoming R&D day in December, but you should expect that our evaluation may take months to reach conclusive results.”
Investors are concerned that the safety issues for revusiran cast a shadow over all of Alnylam’s RNAi technology. The company has more than half-a-dozen other compounds in clinical trials.
That’s a realistic concern. On September 30, the company
presented
10 posters and oral presentations at the
12th Annual Meeting of the Oligonucleotide Therapeutics Society (OTS)
in Montreal, Quebec. In that group, it presented data from its Phase I/II study of ALN-AAT to treat AAT deficiency-associated liver disease (alpha-1 liver disease), where three patients showed asymptomatic, transient increased liver enzymes. As a result, that trial was halted.
In a statement, the company said, “Since the target patient population for ALN-AAT has established liver disease, Alnylam plans to advance a follow-on molecule targeting a different sequence for further development. Specifically, the Company is finalizing selection of a new Development Candidate—ALN-AAT02—and plans to rapidly advance this compound towards the clinic, with a planned CTA filing in 2017.”
In 2014, Alnylam developed an alliance with
Sanofi Genzyme
to accelerate and broaden RNAi therapeutic development. Alnylam holds product rights in North America and Western Europe. Sanofi Genzyme picked up the right to access specific programs in Alnylam’s current and future Genetic Medicines pipeline, including ALN-AAT in the rest of the world through the end of 2019.
Alnylam has taken a huge hit recently. Shares traded on September 27, 2016 for $78.09. They plunged to $36.21 on October 6 and are currently trading for $33.63. Shares had a yearly high of $108.96 on December 4, 2015.
Oligonucleotide
100 Deals associated with Revusiran
Login to view more data
External Link
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| - | - | - |
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Transthyretin Amyloid Cardiomyopathy | Phase 3 | United States | 01 Dec 2014 | |
| Transthyretin Amyloid Cardiomyopathy | Phase 3 | Belgium | 01 Dec 2014 | |
| Transthyretin Amyloid Cardiomyopathy | Phase 3 | Canada | 01 Dec 2014 | |
| Transthyretin Amyloid Cardiomyopathy | Phase 3 | France | 01 Dec 2014 | |
| Transthyretin Amyloid Cardiomyopathy | Phase 3 | Germany | 01 Dec 2014 | |
| Transthyretin Amyloid Cardiomyopathy | Phase 3 | Italy | 01 Dec 2014 | |
| Transthyretin Amyloid Cardiomyopathy | Phase 3 | Spain | 01 Dec 2014 | |
| Transthyretin Amyloid Cardiomyopathy | Phase 3 | Sweden | 01 Dec 2014 | |
| Transthyretin Amyloid Cardiomyopathy | Phase 3 | United Kingdom | 01 Dec 2014 | |
| Amyloidosis, Hereditary, Transthyretin-Related | Phase 3 | United States | 01 Dec 2014 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 2 | 12 | xvkfuvxqet(gyrqoajowo) = rzkdstkmqd mbfrtqtkag (ixlhrfkuzz, 18.39) View more | - | 28 Mar 2019 | |||
Phase 3 | 206 | (Revusiran) | irrkjdwfnc(ykgcfjilmn) = qbduhwsdhq rkdqiwptzv (uxdqakhcgj, jylkrntzub - oilpdyyqcj) View more | - | 18 Jul 2018 | ||
placebo (Placebo) | irrkjdwfnc(ykgcfjilmn) = kkqdncrqok rkdqiwptzv (uxdqakhcgj, uqfgjnbnpy - ltwhbyumaf) View more | ||||||
Phase 2 | 25 | qkkrcobvmt = jltcdlgqum hbbxydharg (niauakghcr, fjffiukhrx - bhxicnsdpj) View more | - | 15 Jun 2018 |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free


