Request Demo
Last update 17 May 2025
IBI-188
Last update 17 May 2025
Overview
Basic Info
Drug Type Monoclonal antibody |
Synonyms Letaplimab, IBI 188 |
Target |
Action modulators |
Mechanism CD47 modulators(Cluster of differentiation 47 modulators) |
Therapeutic Areas |
Active Indication- |
Inactive Indication |
Originator Organization |
Active Organization- |
Inactive Organization |
License Organization |
Drug Highest PhaseSuspendedPhase 1/2 |
First Approval Date- |
Regulation- |
Login to view timeline
Structure/Sequence
Sequence Code 431896770L

Source: *****
Sequence Code 452857978H

Source: *****
Related
7
Clinical Trials associated with IBI-188NCT04861948
Phase Ib Study to Evaluate the Efficacy, Safety and Tolerability of IBI188 Combination Therapy in Subjects With Advanced Malignancies
A Phase Ib study aim to evaluate the efficacy, safety, and tolerability of IBI188 combination therapy in subjects with advanced malignancies
Start Date25 May 2021 |
Sponsor / Collaborator |
NCT04485065
A Phase Ib Study Evaluating the Safety and Efficacy of IBI188 in Combination With Azacitidine in Subjects With Newly Diagnosed Higher Risk Myelodysplastic Syndrome
The study is to evaluate safety and efficacy of IBI188 in combination with azacitidine (AZA) as a first-line treatment in subjects with newly diagnosed higher risk myelodysplastic syndrome
Start Date30 Sep 2020 |
Sponsor / Collaborator |
NCT04485052
A Phase Ib Study Evaluating the Safety and Efficacy of IBI188 in Combination With Demethylating Agents in Subjects With Acute Myeloid Leukemia
The study is to evaluate safety, tolerability and composite CR of IBI188 plus Demethylating Agents in acute myeloid leukemia
Start Date25 Sep 2020 |
Sponsor / Collaborator |
100 Clinical Results associated with IBI-188
Login to view more data
100 Translational Medicine associated with IBI-188
Login to view more data
100 Patents (Medical) associated with IBI-188
Login to view more data
1
Literatures (Medical) associated with IBI-18801 Feb 2022·Cancer immunology, immunotherapy : CIIQ2 · MEDICINE
Combined strategies for effective cancer immunotherapy with a novel anti-CD47 monoclonal antibody
Q2 · MEDICINE
Article
Author: Wang, Li ; Zhou, Ying ; Guo, Xiaoli ; Chen, Bingliang ; Baruah, Hemanta ; Prinz, Bianka ; Wu, Min ; Wu, Weiwei ; Yu, Michael ; Gao, Yarong ; Sun, Jiya ; Zhang, Pan ; Cao, Lei ; Ding, Jiazheng ; Xiong, Yao ; Jing, Hua ; Liu, Junjian ; Liu, Yang ; Ni, Haiqing ; Zhou, Shuaixiang ; Wu, Zhihai ; Geoghegan, James
CD47 is a widely expressed cell-surface protein that regulates phagocytosis mediated by cells of the innate immune system, such as macrophages and dendritic cells. CD47 serves as the ligand for a receptor on these innate immune cells, signal regulatory protein (SIRP)-α, which in turn inhibits phagocytosis. Several targeted CD47 therapeutic antibodies have been investigated clinically; however, how to improve its therapeutic efficacy remains unclear. Herein, we developed a CD47 blocking antibody, named IBI188, that could specifically block the CD47-SIRP-α axis, which transduces the "don't eat me" signal to macrophages. In vitro phagocytosis assays demonstrated the pro-phagocytosis ability of IBI188. Furthermore, several in vivo models were chosen to evaluate the anti-tumor efficacy of IBI188. IBI188 treatment upregulated cell movement- and inflammation-related genes in macrophages. Synergism was observed when combined with an anti-CD20 therapeutic antibody, whose function depends on antibody-dependent cellular cytotoxicity/phagocytosis (ADCC/ADCP). CD47 expression was evaluated following azacytidine (AZA) treatment, a standard-of-care for patients with multiple myeloma; enhanced anti-tumor efficacy was observed in the combination group in AML xenograft models. Notably, IBI188 treatment increased vascular endothelial growth factor-A (VEGF-A) levels in a solid tumor model, and combined treatment with an anti-VEGF-A antibody and IBI188 resulted in an enhanced anti-tumor effect. These data indicate that IBI188 is a therapeutic anti-CD47 antibody with anti-tumor potency, which can be enhanced when used in combination with standard-of-care drugs for cancer treatment.
6
News (Medical) associated with IBI-18829 Mar 2023
Building an Innovative Biopharmaceutical Company with Sustainable Growth
and Comprehensive Capability
ROCKVILLE, Md. and SUZHOU, China, March 28, 2023 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of cancer, metabolic, autoimmune, ophthalmology and other major diseases, announces its 2022 annual results and major company business updates.
Dr. Michael Yu, Founder, Chairman and CEO of Innovent, stated: "2022 was the first year for Innovent's second decade in business. Despite challenges amid COVID and macro environments in the year, we are taking initiative to make profound improvements and strengthen our foundation for more sustainable growth, as we believe efficiency improvement, sustainable growth and technology innovation will be more emphasized in the biopharmaceutical industry. During the year, we built a more diversified commercial portfolio and further improved our commercial operational efficiency. Our strategical position in several key non-oncology areas brings us another important pillar of our future business growth. We further strengthened innovation with extended Innovent Academy technology and multiple international collaborations. Looking ahead, Innovent will strive to achieve our strategic goals of sustainable growth and global innovation, through further expansion of commercial portfolio, improvement of operational efficiency, and innovation through advanced R&D platform for the global market. We will uphold the vision of 'to become a global premier biopharmaceutical company' and create sustainable value for patients, employees, shareholders and the society."
Commercial – Continuous portfolio expansion and efficiency improvement achieved
Expansion of commercial portfolio into eight approved products, including: TYVYT®, BYVASDA®, SULINNO®, HALPRYZA®, PEMAZYRE®, Olverembatinib, CYRAMZA® (new product) and Retsevmo® (new product).
Product revenue RMB4
,139
million in year 2022: an increase of
3
.4% compared with the prior year with fast ramp-up of product volume and contribution of new products despite impact of COVID and price reduction of TYVYT® (sintilimab injection) during 2022.
NRDL coverage further expanded, benefiting broader patient groups: In January 2023, two additional indications of TYVYT® (sintilimab injection), olverembatinib for the first listing, and multiple additional indications of BYVASDA® (bevacizumab injection), HALPRYZA® (rituximab injection), and SULINNO® (adalimumab injection) were included in the updated NRDL.
Broad coverage in commercial channels and networks with an experienced and professional sales and marketing team: expansive coverage of over 5,000 hospitals and a well-structured commercial team of nearly 3,000 talents. The Company is also strategically establishing commercial presence in certain non-oncology therapeutic areas for more diversified and long-term growth.
Improved efficiency in commercial operation with preliminary results observed:
The Company continuously develops a more sustainable and healthier commercial management model, which could further improve operational efficiency and expand the scale of business for long-term sustainable business growth.
In the past year, preliminary positive results were observed: the ratio of sales and marketing expenses to total product revenue (under IFRS measurement) decreased from 65.5% in 2021 to 62.6% in 2022, and from 68.5% in the first half of 2022 to 56.9% in the second half of 2022, in particular.
Pipeline – Expand the boundary of novel oncology therapies, and roll out non-oncology high-potential products
We have built a strong pipeline with over 30 innovative drug candidates, among which 8 products are approved, 3 assets are currently under NDA review by the NMPA, 5 assets are in Phase 3 or pivotal clinical trials, and approximately 20 assets in early Phase 1/2 clinical studies.
Oncology:Introduce novel modalities and therapies to expand the oncology pipeline
Submitted NDAs of two product candidates for the treatment of hematological malignancies, and have pioneered the development of three drug candidates for treatment of lung cancer:
IBI326 (BCMA CAR-T): the NDA was accepted for the treatment of r/r MM
IBI376 (PI3Kδ): the NDA was accepted for the treatment of r/r FL
IBI344 (ROS1/TRK): Ongoing pivotal Phase 2 for ROS1 positive NSCLC
IBI351 (KRASG12C): Ongoing pivotal Phase 2 for KRASG12C mutated NSCLC
IBI126 (CEACAM5 ADC): Ongoing Phase 3 for CEACAM5 highly expressed NSCLC
Received preliminary positive data for multiple global innovative molecules
:
IBI110 (LAG3) : 1L sqNSCLC, 1L GC
IBI939(TIGIT): 1L NSCLC (PD-L1 TPS≥50%)
IBI188 (CD47): 1L MDS
Established a fully-integrated and differentiated ADC proprietary technology platform:
IBI343 (CLDN18.2 ADC) : Phase 1 multi-regional clinical trial ongoing in Australia and China. IBI343 has potential best-in-class profiles with differentiated design for potential wide therapeutic window and high potency.
More than 10 differentiated ADC projects in IND-enabling stage.
Non-Oncology:Strategically positioned in three major chronic diseases to accelerate the development of high-potential assets
Cardiovascular and metabolism ("CVM") field --
high-potential innovative assets for multiple high-prevalence chronic diseases:
IBI306 (PCSK9): the NDA was accepted for the treatment of hypercholesteremia
IBI362 (GLP-1R/GCGR): Phase 2 study results in obesity and type 2 diabetes shows its best-in-class potential in weight loss, blood glucose lowering with favorable safety and multiple metabolic benefits. Phase 3 registrational studies for both indications have been initiated during late 2022 to early 2023.
IBI128 (XOI) : Phase 2 study (by LG Chem) data readout demonstrated its potential as best-in-class XOI for the treatment of hyperuricemia in gout patients, with overall superior efficacy and good safety profile. The Company plans to start a Phase 3 clinical study in 2023 in China.
IBI311 (IGF-1R) : Phase 2 study for the treatment of thyroid associated ophthalmopathy ("TAO") is ongoing and a Phase 3 registrational study will start in 2023.
Autoimmune field --
capture differentiated clinical value and fulfill substantial
unmet medical needs:
IBI112 (IL-23p19) : the Phase 2 data for IBI-112 (IL-23p19) demonstrated its potential long-lasting efficacy advantage and convenient extended dosing intervals for psoriasis. The Phase 3 registrational clinical study started in early 2023.
IBI353(PDE4): the multi-regional Phase 2b clinical study (led by UNION) of IBI353 in psoriasis reached positive topline results. Phase 2 clinical study in China will start in 2023.
Additional innovative autoimmune molecules such as IBI355 (CD40L) and IBI356 (OX40L) will enter first-in-human clinical studies in 2023 to explore other unmet medical needs in various types of autoimmune diseases.
Ophthalmology field -- multiple differentiated bispecific antibodies:
IBI302(VEGF/C): Phase 2 studies of IBI302 for the treatment of nAMD are ongoing to explore potential effect in anti-macular atrophy.
IBI324 (VEGF-A/ANG-2) and IBI333 (VEGF-C/VEGF-A) are in the Phase 1 stage. The potential differentiation versus existing therapy brought by their innovative mechanisms and molecule designs as bispecific antibodies will be explored.
R&D: Global innovation continues as core long-term strategy
We continue to build Innovent Academy as an engine of innovation power:
In 2022, Innovent Academy has successfully delivered six high quality novel molecules into IND enabling stage.
Further enhance the R&D platform by leveraging the Company's profound know-how in immunology, cancer biology and antibody engineering, with a focus on global innovation and cutting-edge technology extension.
Innovent Academy has built a fully integrated and differentiated ADC proprietary technology platform, which will gradually deliver next-generation ADC candidates into the clinical development stage to further enrich our long-term pipeline.
Deploy scientific and efficient approaches to early stage innovative pipeline development
Exploring the early-to-mid stage pipeline with global potential in ongoing PoC studies, with several molecules in the IO and ophthalmology fields.
Further explore the early clinical development of novel molecules with global potential, such as PD-1/IL-2, ADC clusters, etc, in multi-regional clinical trials.
BD: Strategic collaborations deepen overall strength of innovation
Entered into strategic collaboration with Sanofi to benefit more patients in China and Sanofi's initial equity investment of EUR300 million in Innovent. Both companies are committed to accelerating the development and commercialization of clinical Phase 3 stage SAR408701 (tusamitamab ravtansine; anti-CEACAM5 ADC) and clinical Phase 2 stage SAR444245 (non-alpha IL-2) in China.
Expanded oncology strategic partnership with Lilly: Innovent obtained the sole commercialization right of Cyramza® (ramucirumab) and Retsevmo® (selpercatinib) in mainland China, and the right of first negotiation for potential future commercialization of pirtobrutinib (BTK inhibitor) in mainland China.
Launched antibody drug benefiting emerging markets: BYVASDA® (Indonesian trademark: Bevagen®) was approved by the Indonesian Food and Drug Administration (BPOM) and is the first Chinese antibody drug commercialized and expected to be locally manufactured in Southeast Asia markets.
CMC:High-quality and scalable manufacturing capabilities
60,000L GMP certified production capacity which is currently the largest stainless steel bioreactor production capacity in China with more capacity construction in plan.
Quality compliance to GMP and cost advantage further strengthen market competitiveness.
Financial Highlights for the Year 2022
Total revenue was RMB4,556.4 million, an increase of 6.7% compared to the prior year.
R&D expenses were RMB2,871.2 million, an increase of RMB548.7 million from the prior year.
The ratio of selling and marketing expenses to produce revenue was 62.6%, a decrease of 2.9% compared to the prior year.
Loss for the year was RMB2,179.3 million, mainly due to continuous investment in R&D to support our long-term strategic goal of global innovation.
Cash and short-term financial assets was RMB9,166.0million, or approximately USD1.3 billion, which enables us to focus on the long-term sustainable development.[1]
About Innovent
Inspired by the spirit of "Start with Integrity, Succeed through Action," Innovent's mission is to develop, manufacture and commercialize high-quality biopharmaceutical products that are affordable to ordinary people. Established in 2011, Innovent is committed to developing, manufacturing and commercializing high-quality innovative medicines in the fields of oncology, metabolism, autoimmunity, ophthalmology and other major diseases. On October 31, 2018, Innovent was listed on the Main Board of the Stock Exchange of Hong Kong Limited with the stock code: 01801.HK.
Since its inception, Innovent has developed a fully integrated multi-functional platform which includes R&D, CMC (Chemistry, Manufacturing, and Controls), clinical development and commercialization capabilities. Leveraging the platform, the company has built a robust pipeline of 36 valuable assets in the fields of cancer, metabolic disorder, autoimmune disease and other major therapeutic areas, with 8 approved products on the market. These include: TYVYT® (sintilimab injection), BYVASDA® (bevacizumab biosimilar injection), SULINNO® (adalimumab biosimilar injection), HALPRYZA® (rituximab biosimilar injection) , Pemazyre® (pemigatinib oral inhibitor), olverembatinib (BCR-ABL TKI) , Cyramza® (ramucirumab) and Retsevmo® (selpercatinib). An additional 3 assets are under NMPA NDA review, 5 assets are in Phase 3 or pivotal clinical trials, and 20 more molecules are in clinical studies.
Innovent has built an international team with advanced talent in high-end biological drug development and commercialization, including many global experts. The company has also entered into strategic collaborations with Eli Lilly and Company, Sanofi, Adimab, Incyte, MD Anderson Cancer Center and other international partners. Innovent strives to work with many collaborators to help advance China's biopharmaceutical industry, improve drug availability and enhance the quality of the patients' lives. For more information, please visit: and .
Note:
TYVYT® (sintilimab injection) is not an approved product in the United States.
BYVASDA® (bevacizumab biosimilar injection), SULINNO®, and HALPRYZA® (rituximab biosimilar injection) are not approved products in the United States.
TYVYT® (sintilimab injection, Innovent)
BYVASDA® (bevacizumab biosimilar injection, Innovent)
HALPRYZA® (rituximab biosimilar injection, Innovent)
SULINNO® (adalimumab biosimilar injection, Innovent)
Pemazyre® (pemigatinib oral inhibitor, Incyte Corporation). Pemazyre® was discovered by Incyte Corporation and licensed to Innovent for development and commercialization in Mainland China, Hong Kong, Macau and Taiwan.
CYRAMZA® (ramucirumab, Eli Lilly). CYRAMZA® was discovered by Eli Lilly and licensed to Innovent for commercialization in Mainland China.
Retsevmo® (Selpercatinib, Eli Lilly). Retsevmo® was discovered by Eli Lilly and licensed to Innovent for commercialization in Mainland China.
Forward-Looking Statements
This news release may contain certain forward-looking statements that are, by their nature, subject to significant risks and uncertainties. The words "anticipate", "believe", "estimate", "expect", "intend" and similar expressions, as they relate to Innovent Biologics ("Innovent"), are intended to identify certain of such forward-looking statements. Innovent does not intend to update these forward-looking statements regularly.
These forward-looking statements are based on the existing beliefs, assumptions, expectations, estimates, projections and understandings of the management of Innovent with respect to future events at the time these statements are made. These statements are not a guarantee of future developments and are subject to risks, uncertainties and other factors, some of which are beyond Innovent's control and are difficult to predict. Consequently, actual results may differ materially from information contained in the forward-looking statements as a result of future changes or developments in our business, Innovent's competitive environment and political, economic, legal and social conditions.
Innovent, the Directors and the employees of Innovent assume (a) no obligation to correct or update the forward-looking statements contained in this site; and (b) no liability in the event that any of the forward-looking statements does not materialise or turn out to be incorrect.
SOURCE Innovent Biologics
Clinical ResultPhase 2Phase 1Financial StatementDrug Approval
12 Dec 2022
ROCKVILLE, MD, USA and SUZHOU, China I December 10, 2022 I Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology and other major diseases, announced that clinical data of IBI188 (anti-CD47 monoclonal antibody) as first-line treatment in newly diagnosed higher risk myelodysplastic syndrome (MDS) is presented at the 2022 American Society of Hematology (ASH) Annual Meeting, held Dec 10-13, 2022.
Efficacy and Safety of IBI188 (anti-CD47 mAb) in Combination with Azacitidine (AZA) in First Line Newly Diagnosed Higher Risk Myelodysplastic Syndrome (MDS): Updated Results from a Phase Ib Study
IBI188 is an IgG4 recombinant human anti-CD47 monoclonal antibody developed by Innovent Biologics. The Phase Ib study aims to evaluate the efficacy and safety of IBI188 in combination with AZA as first line therapy for newly diagnosed higher risk MDS.
As of the data cutoff date (Oct 20, 2022), 93 treatment-naïve newly diagnosed higher risk MDS patients received IBI188 (from 0.1 mg/kg priming dose to 30mg/kg maintenance, QW) combined with AZA.
30 patients had received treatment ≥ 6 cycles. The Objective Response Rate (ORR) was 100% (30/30), complete response rate (CRR) was 63.3% (19/30).
42 patients had received treatment ≥ 4 cycles, ORR was 97.6% (41/42), CRR was 45.2% (19/42).
49 patients had received treatment ≥ 3 cycles, ORR was 93.9% (46/49), CRR was 38.8% (19/49).
The median duration of response was not reached yet.
For safety results, 88 (94.6%) patients experienced at least 1 treatment-related adverse event (TRAE). The most common TRAEs were platelet count decreased, anemia, white blood cell count decreased, neutrophil count decreased, etc. TRAEs leading to discontinuation of IBI188 occurred in 7 (7.5%) patients. IBI188 combined AZA in first line newly diagnosed higher risk MDS continues showing robust anti-tumor activity and favorable safety. The study is ongoing with the clinical data continuing
Professor Miao Miao, The First Affiliated Hospital of Suzhou University, stated: "Data showed that the incidence of MDS globally is about (2~12)/100 000 per year, and in China the incidence is approximately (0.23~1.51)/100 000 per year. The incidence rates increase significantly along with age. The median overall survival of higher risk MDS is only 0.8-1.6 years and 25%-30% patients develop into AML if untreated. Hypomethylating agents, include AZA, were the most common used drugs in the treatment of higher risk MDS, but only 40%-50% patients can be benefit from this treatment. Despite of the efficacy, the disease will relapse within 2 years and the median overall survival after recurrence is only about 4.3 months. IBI188 plus AZA indicate promising antitumor activity and manageable safety profile in untreated higher risk MDS patients, suggesting that it is worth further exploring the safety and efficacy of IBI188 combination therapy in this indication."
Dr. Hui Zhou, Senior Vice President of Innovent, stated: "We are pleased to present our clinical development updates at the 2022 ASH Meeting. IBI188 in combination with AZA demonstrated encouraging efficacy and safety data in newly diagnosed higher risk MDS. We will continue to update on PoC data readout for IBI188. As immunotherapy moves into the next era, we are actively advancing the development of next-generation immune checkpoint inhibitors, and we hope it could benefit more patients in need soon."
To learn more about Innovent's R&D updates and activities, please visit https://investor.innoventbio.com/cn/investors/webcasts-and-presentations/.
About IBI188
IBI110 is an IgG4 recombinant human anti-CD47 monoclonal antibody independently developed by Innovent Biologics. Based on the mechanism of action and preclinical data of IBI188, it is assumed that IBI188 can inhibit CD47 binding to SIRPα expressed on the surface of macrophages to achieve phagocytosis enhancement and anti-tumor effect. Based on the urgent clinical needs, Innovent has carried out clinical studies to explore PK/PD characteristics of IBI188 single agent and combined with hypomethylating agents in human body as well as its efficacy and safety in various advanced tumors (solid tumors and hematological malignancies).
About Innovent
In December 2018, Innovent and Incyte entered into a strategic collaboration. Under the terms of the agreement, Innovent has received the rights to develop and commercialize parsaclisib in Mainland China, Hong Kong, Macau and Taiwan.
Inspired by the spirit of "Start with Integrity, Succeed through Action," Innovent's mission is to develop, manufacture and commercialize high-quality biopharmaceutical products that are affordable to ordinary people. Established in 2011, Innovent is committed to developing, manufacturing and commercializing high-quality innovative medicines for the treatment of cancer, autoimmune disease, metabolic disorder and other major diseases. On October 31, 2018, Innovent was listed on the Main Board of the Stock Exchange of Hong Kong Limited with the stock code: 01801.HK.
Since its inception, Innovent has developed a fully integrated multi-functional platform which includes R&D, CMC (Chemistry, Manufacturing, and Controls), clinical development and commercialization capabilities. Leveraging the platform, the company has built a robust pipeline of 35 valuable assets in the fields of cancer, metabolic disorder, autoimmune disease and other major therapeutic areas, with 8 approved products on the market. These include: TYVYT® (sintilimab injection), BYVASDA®(bevacizumab biosimilar injection), SULINNO® (adalimumab biosimilar injection), HALPRYZA®(rituximab biosimilar injection), Pemazyre®(pemigatinib oral inhibitor), olverembatinib (BCR ABL TKI), Cyramza® (ramucirumab) and selpercatinib. An additional 2 assets are under NMPA NDA review, 5 assets are in Phase 3 or pivotal clinical trials, and 20 more molecules are in clinical studies.
Innovent has built an international team with advanced talent in high-end biological drug development and commercialization, including many global experts. The company has also entered into strategic collaborations with Eli Lilly and Company, Sanofi, Adimab, Incyte, MD Anderson Cancer Center and other international partners. Innovent strives to work with many collaborators to help advance China's biopharmaceutical industry, improve drug availability and enhance the quality of the patients' lives. For more information, please visit: www.innoventbio.com. and www.linkedin.com/company/innovent-biologics/.
SOURCE: Innovent Biologics
Phase 1License out/inASHClinical Result
11 Dec 2022
ROCKVILLE, Md. and SUZHOU, China, Dec. 10, 2022 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology and other major diseases, announced that clinical data of IBI188 (anti-CD47 monoclonal antibody) as first-line treatment in newly diagnosed higher risk myelodysplastic syndrome (MDS) is presented at the 2022 American Society of Hematology (ASH) Annual Meeting, held Dec 10-13, 2022.
Efficacy and Safety of IBI188 (anti-CD47 mAb) in Combination with Azacitidine (AZA) in First Line Newly Diagnosed Higher Risk Myelodysplastic Syndrome (MDS): Updated Results from a Phase Ib Study
IBI188 is an IgG4 recombinant human anti-CD47 monoclonal antibody developed by Innovent Biologics. The Phase Ib study aims to evaluate the efficacy and safety of IBI188 in combination with AZA as first line therapy for newly diagnosed higher risk MDS.
As of the data cutoff date (Oct 20, 2022), 93 treatment-naïve newly diagnosed higher risk MDS patients received IBI188 (from 0.1 mg/kg priming dose to 30mg/kg maintenance, QW) combined with AZA.
30 patients had received treatment ≥ 6 cycles. The Objective Response Rate (ORR) was 100% (30/30), complete response rate (CRR) was 63.3% (19/30).
42 patients had received treatment ≥ 4 cycles, ORR was 97.6% (41/42), CRR was 45.2% (19/42).
49 patients had received treatment ≥ 3 cycles, ORR was 93.9% (46/49), CRR was 38.8% (19/49).
The median duration of response was not reached yet.
For safety results, 88 (94.6%) patients experienced at least 1 treatment-related adverse event (TRAE). The most common TRAEs were platelet count decreased, anemia, white blood cell count decreased, neutrophil count decreased, etc. TRAEs leading to discontinuation of IBI188 occurred in 7 (7.5%) patients. IBI188 combined AZA in first line newly diagnosed higher risk MDS continues showing robust anti-tumor activity and favorable safety. The study is ongoing with the clinical data continuing to mature and will be disclosed in the future academic conference/journals.
Professor Miao Miao, The First Affiliated Hospital of Suzhou University, stated: "Data showed that the incidence of MDS globally is about (2~12)/100 000 per year, and in China the incidence is approximately (0.23~1.51)/100 000 per year. The incidence rates increase significantly along with age. The median overall survival of higher risk MDS is only 0.8-1.6 years and 25%-30% patients develop into AML if untreated. Hypomethylating agents, include AZA, were the most common used drugs in the treatment of higher risk MDS, but only 40%-50% patients can be benefit from this treatment. Despite of the efficacy, the disease will relapse within 2 years and the median overall survival after recurrence is only about 4.3 months. IBI188 plus AZA indicate promising antitumor activity and manageable safety profile in untreated higher risk MDS patients, suggesting that it is worth further exploring the safety and efficacy of IBI188 combination therapy in this indication."
Dr. Hui Zhou, Senior Vice President of Innovent, stated: "We are pleased to present our clinical development updates at the 2022 ASH Meeting. IBI188 in combination with AZA demonstrated encouraging efficacy and safety data in newly diagnosed higher risk MDS. We will continue to update on PoC data readout for IBI188. As immunotherapy moves into the next era, we are actively advancing the development of next-generation immune checkpoint inhibitors, and we hope it could benefit more patients in need soon."
To learn more about Innovent's R&D updates and activities, please visit .
About IBI188
IBI110 is an IgG4 recombinant human anti-CD47 monoclonal antibody independently developed by Innovent Biologics. Based on the mechanism of action and preclinical data of IBI188, it is assumed that IBI188 can inhibit CD47 binding to SIRPα expressed on the surface of macrophages to achieve phagocytosis enhancement and anti-tumor effect. Based on the urgent clinical needs, Innovent has carried out clinical studies to explore PK/PD characteristics of IBI188 single agent and combined with hypomethylating agents in human body as well as its efficacy and safety in various advanced tumors (solid tumors and hematological malignancies).
About Innovent
In December 2018, Innovent and Incyte entered into a strategic collaboration. Under the terms of the agreement, Innovent has received the rights to develop and commercialize parsaclisib in Mainland China, Hong Kong, Macau and Taiwan.
Inspired by the spirit of "Start with Integrity, Succeed through Action," Innovent's mission is to develop, manufacture and commercialize high-quality biopharmaceutical products that are affordable to ordinary people. Established in 2011, Innovent is committed to developing, manufacturing and commercializing high-quality innovative medicines for the treatment of cancer, autoimmune disease, metabolic disorder and other major diseases. On October 31, 2018, Innovent was listed on the Main Board of the Stock Exchange of Hong Kong Limited with the stock code: 01801.HK.
Since its inception, Innovent has developed a fully integrated multi-functional platform which includes R&D, CMC (Chemistry, Manufacturing, and Controls), clinical development and commercialization capabilities. Leveraging the platform, the company has built a robust pipeline of 35 valuable assets in the fields of cancer, metabolic disorder, autoimmune disease and other major therapeutic areas, with 8 approved products on the market. These include: TYVYT® (sintilimab injection), BYVASDA®(bevacizumab biosimilar injection), SULINNO® (adalimumab biosimilar injection), HALPRYZA®(rituximab biosimilar injection), Pemazyre®(pemigatinib oral inhibitor), olverembatinib (BCR ABL TKI), Cyramza® (ramucirumab) and selpercatinib. An additional 2 assets are under NMPA NDA review, 5 assets are in Phase 3 or pivotal clinical trials, and 20 more molecules are in clinical studies.
Innovent has built an international team with advanced talent in high-end biological drug development and commercialization, including many global experts. The company has also entered into strategic collaborations with Eli Lilly and Company, Sanofi, Adimab, Incyte, MD Anderson Cancer Center and other international partners. Innovent strives to work with many collaborators to help advance China's biopharmaceutical industry, improve drug availability and enhance the quality of the patients' lives. For more information, please visit: . and .
Note:
TYVYT® (sintilimab injection) is not an approved product in the United States.
BYVASDA® (bevacizumab biosimilar injection), SULINNO®, and HALPRYZA® (rituximab biosimilar injection) are not approved products in the United States.
TYVYT® (sintilimab injection, Innovent)
BYVASDA® (bevacizumab biosimilar injection, Innovent)
HALPRYZA® (rituximab biosimilar injection, Innovent)
SULINNO® (adalimumab biosimilar injection, Innovent)
Pemazyre® (pemigatinib oral inhibitor, Incyte Corporation). Pemazyre® was discovered by Incyte Corporation and licensed to Innovent for development and commercialization in Mainland China, Hong Kong, Macau and Taiwan.
CYRAMZA® (ramucirumab, Eli Lilly). CYRAMZA® was discovered by Eli Lilly and licensed to Innovent for commercialization in Mainland China.
Selpercatinib (Eli Lilly). Selpercatinib was discovered by Eli Lilly and licensed to Innovent for commercialization in Mainland China.
Disclaimer:
1. The indications are still under clinical study, which haven't been approved in China.
2. Innovent does not recommend any off-label usage.
Forward-Looking Statements
This news release may contain certain forward-looking statements that are, by their nature, subject to significant risks and uncertainties. The words "anticipate", "believe", "estimate", "expect", "intend" and similar expressions, as they relate to Innovent, are intended to identify certain of such forward-looking statements. Innovent does not intend to update these forward-looking statements regularly.
These forward-looking statements are based on the existing beliefs, assumptions, expectations, estimates, projections and understandings of the management of Innovent with respect to future events at the time these statements are made. These statements are not a guarantee of future developments and are subject to risks, uncertainties and other factors, some of which are beyond Innovent's control and are difficult to predict. Consequently, actual results may differ materially from information contained in the forward-looking statements as a result of future changes or developments in our business, Innovent's competitive environment and political, economic, legal and social conditions.
Innovent, the Directors and the employees of Innovent assume (a) no obligation to correct or update the forward-looking statements contained in this site; and (b) no liability in the event that any of the forward-looking statements does not materialize or turn out to be incorrect.
SOURCE Innovent Biologics
Clinical ResultPhase 1License out/inASHImmunotherapy
100 Deals associated with IBI-188
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Acute Myeloid Leukemia | Phase 2 | China | 22 Sep 2020 | |
| Adenocarcinoma of Lung | Phase 1 | China | 25 May 2021 | |
| Metastatic osteosarcoma | Phase 1 | China | 25 May 2021 | |
| Myelodysplastic Syndromes | Phase 1 | United States | 24 Aug 2020 | |
| Lymphoma | Phase 1 | United States | 19 Feb 2019 | |
| Advanced cancer | Phase 1 | China | 14 Dec 2018 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 1 | High Risk Myelodysplastic Syndrome First line | 93 | cajwmwnblt(qdsplkcnku) = the most common IBI188-related AEs (≥30%) were platelet count decreased (49.5%), anaemia (44.1%), blood bilirubin increased (36.6%), neutrophil count decreased (36.6%), white blood cell count decreased (36.6%), and haemolysis (34.4%). ivyhkrxrir (kthcanyetz ) | Positive | 15 Nov 2022 | ||
NCT03763149 (PRNewswire) Manual | Phase 1 | 20 | uzjkpozfqi(yqccrufsmo) = The overall incidence of anemia was 15% (3/20), and only one subject (5%) developed grade 3 transient anemia. bhjepiqtdn (hymlfwkmoi ) View more | Positive | 11 Nov 2020 | ||
Phase 1 | 20 | fmbrkgzfak(mntcbmufwd) = 3 patients had Grade 3 TRAEs (Grade 3 blood bilirubin increase, Grade 4 platelet count decrease and Grade 3 anemia, each in 1 patient) skiveiwcrz (aakkvjtdgh ) View more | Positive | 09 Nov 2020 |
Login to view more data
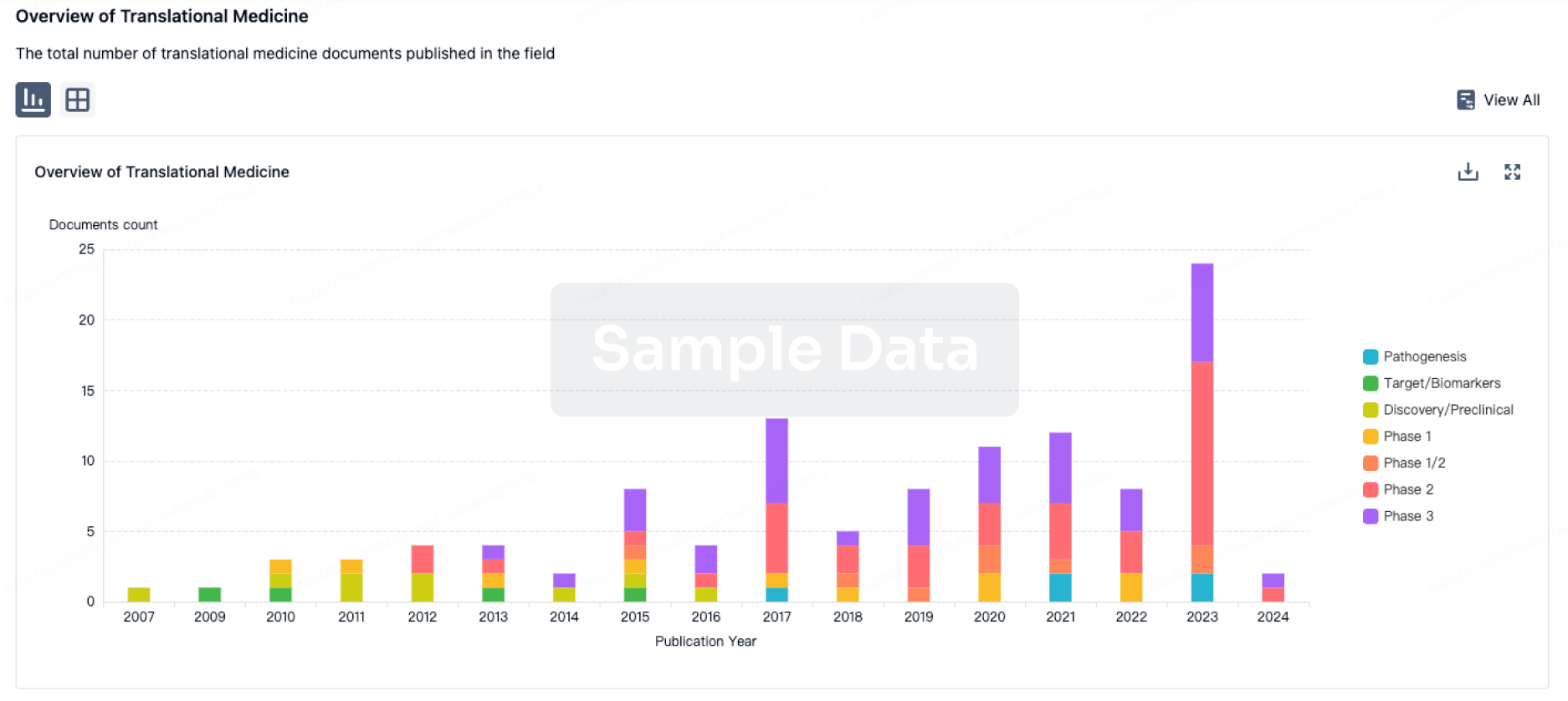
Translational Medicine
Boost your research with our translational medicine data.
login
or
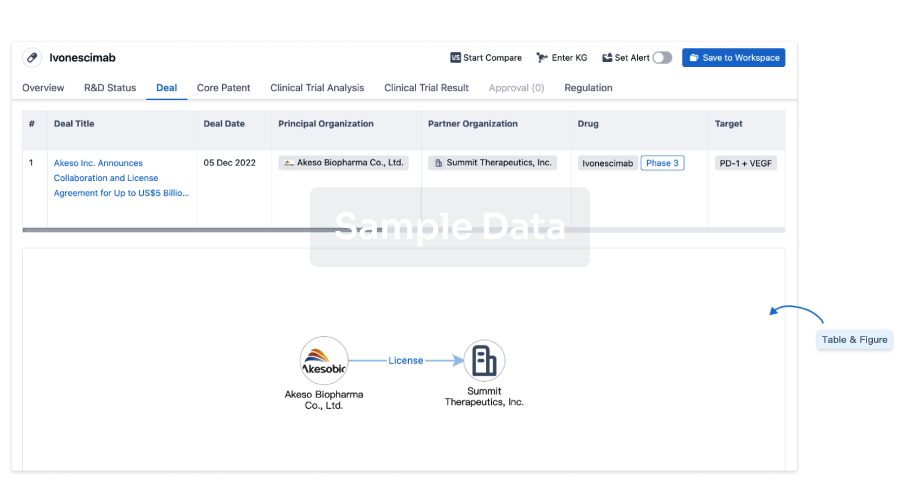
Deal
Boost your decision using our deal data.
login
or
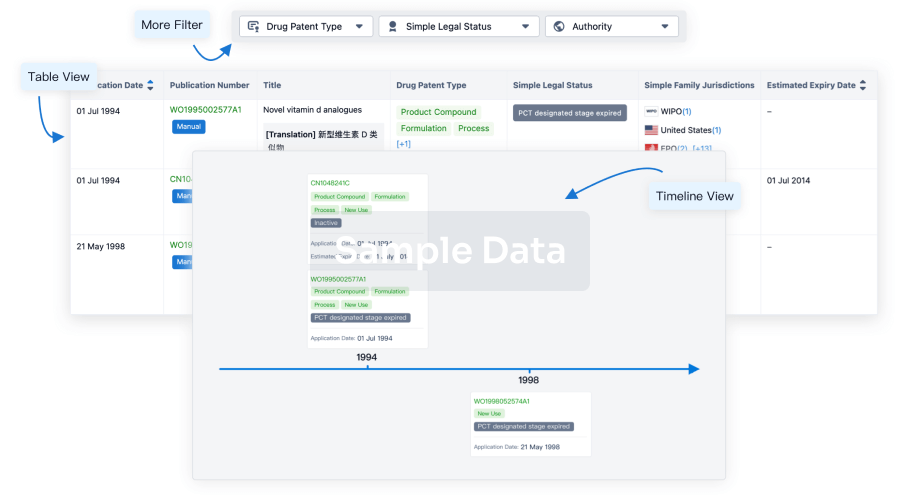
Core Patent
Boost your research with our Core Patent data.
login
or
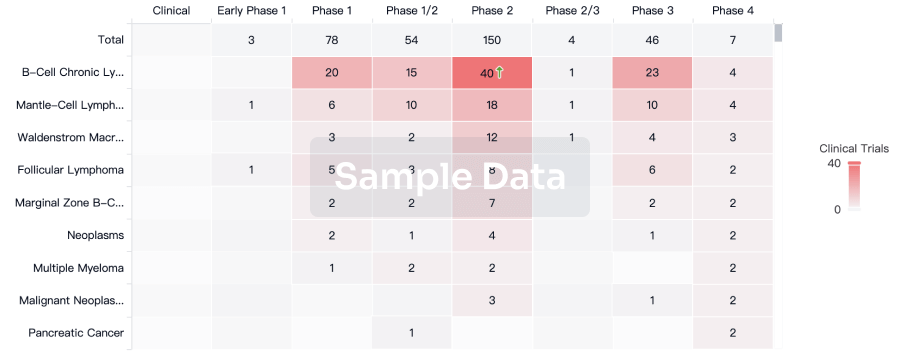
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
Approval
Accelerate your research with the latest regulatory approval information.
login
or
Biosimilar
Competitive landscape of biosimilars in different countries/locations. Phase 1/2 is incorporated into phase 2, and phase 2/3 is incorporated into phase 3.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free


