QALSODY approved for SOD1-ALS; MAA under review in EU
Reported positive Phase 3 eplontersen ATTRv-PN data; December 22, 2023 PDUFA date
On track to achieve 2023 financial guidance
CARLSBAD, Calif., May 3, 2023 /PRNewswire/ -- Ionis Pharmaceuticals, Inc. (Nasdaq: IONS) (the "Company"), today reported financial results for the first quarter of 2023. Financial results are summarized below:
Financial Highlights
Revenue for the first quarter of 2023 was in line with expectations and included revenue from numerous diverse sources
Operating expenses increased in the first quarter of 2023 compared to the prior year as planned, reflecting investments in advancing Ionis' pipeline, technology and go-to-market activities for eplontersen, olezarsen and donidalorsen
Cash and short-term investments of $2.3 billion at March 31, 2023 enables continued investment in creating future growth opportunities
Reaffirmed 2023 financial guidance
"2023 is off to a strong start. With QALSODY's approval, it joins SPINRAZA as a new groundbreaking medicine to treat a devastating neurological disease, further validating our RNA-targeting therapeutic platform. We also achieved another important milestone with our recent positive eplontersen Phase 3 data. We believe the positive efficacy and safety data, and the attractive self-administered dosing profile, position eplontersen to be an important treatment for ATTRv-PN patients, who today are underserved. We look forward to the first potential approval of eplontersen in the U.S. in December," said Brett P. Monia, Ph.D., chief executive officer of Ionis. "In addition, we further expanded our industry-leading late-stage pipeline to seven programs across nine indications following the start of GSK's bepirovirsen hepatitis B program. In the second half, we plan to report results from our olezarsen Phase 3 FCS study, which if positive, positions us for our first independent commercial launch. These recent achievements, together with our upcoming milestones, continue to build value for Ionis stakeholders."
Recent Late-Stage Pipeline Highlights
FDA granted Biogen accelerated approval of QALSODY (tofersen) for patients with SOD1-ALS
FDA accepted eplontersen NDA for patients with polyneuropathy caused by hereditary TTR amyloidosis (ATTRv-PN) with a PDUFA date of December 22, 2023
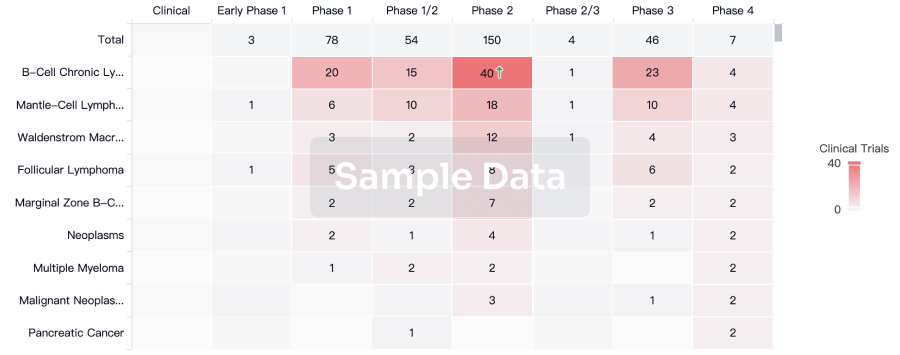
Presented positive week-35 and week-66 data from the Phase 3 NEURO-TTRansform study of eplontersen in patients with ATTRv-PN
GSK advanced bepirovirsen into Phase 3 development in patients with chronic hepatitis B
Recent Additional Pipeline Updates
Biogen presented data demonstrating IONIS-MAPTRx (BIIB080) substantially reduced tau protein in patients with early-stage Alzheimer's disease
Continued to focus R&D efforts by discontinuing two programs that did not meet Ionis' target product profile, cimdelirsen for acromegaly and sapablursen for beta-thalassemia. Ionis continues to advance the Phase 2 sapablursen study for polycythemia vera
First Quarter 2023 Financial Results
"Our first quarter results were in line with our expectations. We generated meaningful revenue while continuing to invest in key growth opportunities across our business. These results keep us on track to achieve our 2023 financial guidance," said Elizabeth L. Hougen, chief financial officer of Ionis. "We plan to continue investing in areas with the greatest potential to drive growth. As such, we expect our investments to grow modestly as we advance and expand our late-stage pipeline and move our near-term commercial opportunities toward the market. Additionally, as we keep more programs for ourselves, we expect a greater proportion of commercial revenues compared to R&D revenues, and our commercial revenues to be the primary driver of future revenue growth."
Revenue
Ionis' revenue was comprised of the following:
Ionis continued to derive its revenue for the first quarter of 2023 from diverse sources, with approximately half coming from commercial products and half from numerous partnered programs. Commercial revenue for the first quarter of 2023 included $50 million from SPINRAZA royalties. Global SPINRAZA product sales of $443 million decreased six percent in the first quarter of 2023, compared to the same period last year primarily due to the impact from foreign currency, fewer new patient starts in the U.S. and channel dynamics.
R&D revenue for the first quarter of 2023 included $24 million from AstraZeneca for its share of the global Phase 3 development costs for eplontersen, $20 million from Biogen for advancing several neurology disease programs and $15 million from GSK for advancing bepirovirsen into Phase 3 development. Already in the second quarter, the Company earned $16 million in a milestone payment from Biogen when QALSODY was approved in the U.S.
Operating Expenses
Ionis' operating expenses increased in the first quarter of 2023 compared to the same period in 2022, consistent with expectations. As Ionis advanced its robust pipeline, study costs increased as most of the Company's Phase 3 studies were either fully enrolled or approaching full enrollment resulting in higher R&D expenses year over year. Additionally, as Ionis prepares to launch eplontersen, olezarsen and donidalorsen, the Company's SG&A expenses also increased year over year.
Balance Sheet
As of March 31, 2023, Ionis' cash, cash equivalents and short-term investments increased to $2.3 billion compared to $2.0 billion at December 31, 2022 primarily due to the $500 million Ionis received from Royalty Pharma in January 2023. Ionis' working capital also increased over the same period primarily due to the Company's higher cash and short-term investments balance. Additionally, the Company recorded a long-term liability for future royalties due to Royalty Pharma in the first quarter of 2023.
Webcast
Management will host a conference call and webcast to discuss Ionis' first quarter 2023 results at 11:30 a.m. Eastern time on Wednesday, May 3, 2023. Interested parties may access the webcast here. A webcast replay will be available for a limited time at the same address. To access the Company's first quarter 2023 earnings slides click here.
About Ionis Pharmaceuticals, Inc.
For more than 30 years, Ionis has been a leader in RNA-targeted therapy, pioneering new markets and changing standards of care. Ionis currently has four marketed medicines and a promising late-stage pipeline highlighted by cardiovascular and neurological franchises. Our scientific innovation began and continues with the knowledge that sick people depend on us, which fuels our vision to become the leader in genetic medicine, utilizing a multi-platform approach to discover, develop and deliver life-transforming therapies.
To learn more about Ionis visit or follow us on Twitter @ionispharma.
Ionis' Forward-looking Statement
This press release includes forward-looking statements regarding Ionis' business, financial guidance and the therapeutic and commercial potential of QALSODY (tofersen), SPINRAZA (nusinersen), TEGSEDI (inotersen), WAYLIVRA (volanesorsen), eplontersen, olezarsen, donidalorsen, ION363, pelacarsen, bepirovirsen, Ionis' technologies and Ionis' other products in development. Any statement describing Ionis' goals, expectations, financial or other projections, intentions or beliefs is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to certain risks and uncertainties including those inherent in the process of discovering, developing and commercializing medicines that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such medicines. Ionis' forward-looking statements also involve assumptions that, if they never materialize or prove correct, could cause its results to differ materially from those expressed or implied by such forward-looking statements. Although Ionis' forward-looking statements reflect the good faith judgment of its management, these statements are based only on facts and factors currently known by Ionis. As a result, you are cautioned not to rely on these forward-looking statements. These and other risks concerning Ionis' programs are described in additional detail in Ionis' annual report on Form 10-K for the year ended December 31, 2022, which is on file with the Securities and Exchange Commission. Copies of this and other documents are available from the Company.
In this press release, unless the context requires otherwise, "Ionis," "Company," "we," "our" and "us" all refer to Ionis Pharmaceuticals and its subsidiaries.
Ionis Pharmaceuticals® is a registered trademark of Ionis Pharmaceuticals, Inc. Akcea Therapeutics® is a registered trademark of Akcea Therapeutics, Inc. TEGSEDI® is a registered trademark of Akcea Therapeutics, Inc. WAYLIVRA® is a registered trademark of Akcea Therapeutics, Inc. QALSODY™ is a trademark of Biogen. SPINRAZA® is a registered trademark of Biogen.
Reconciliation of GAAP to Non-GAAP Basis
As illustrated in the Selected Financial Information in this press release, non-GAAP operating expenses, non-GAAP loss from operations, and non-GAAP net loss were adjusted from GAAP to exclude compensation expense related to equity awards and the related tax effects. Compensation expense related to equity awards are non-cash. These measures are provided as supplementary information and are not a substitute for financial measures calculated in accordance with GAAP. Ionis reports these non-GAAP results to better enable financial statement users to assess and compare its historical performance and project its future operating results and cash flows. Further, the presentation of Ionis' non-GAAP results is consistent with how Ionis' management internally evaluates the performance of its operations.
2023 Key Value Driving Events(1)
SOURCE Ionis Pharmaceuticals, Inc.