Request Demo
Last update 04 Dec 2025
Loncastuximab tesirine
Last update 04 Dec 2025
Overview
Basic Info
Drug Type Antibody drug conjugate (ADC) |
Synonyms Anti-CD19-PBD-conjugate-ADC, Immunoglobulin g1-kappa, anti-(homo sapiens b-lymphocyte antigen cd-19)chimeric monoclonal antibody conjugated to an average of two molecules of tesirine.gamma.1 heavy chain (1-449) (mus musculus vh (ighv1-69*02 (86%) (ighd)-ighj4*01)) (8.8.13) (1-120) -, Lonca + [12] |
Target |
Action inhibitors |
Mechanism CD19 inhibitors(B-lymphocyte antigen CD19 inhibitors), DNA inhibitors(DNA inhibitors) |
Therapeutic Areas |
Inactive Indication |
Originator Organization |
Active Organization |
Inactive Organization- |
License Organization |
Drug Highest PhaseApproved |
First Approval Date United States (23 Apr 2021), |
RegulationPriority Review (United States), Accelerated Approval (United States), Orphan Drug (United States), Priority Review (China), Orphan Drug (Australia), Conditional marketing approval (European Union), Accelerated assessment (United States), Conditional marketing approval (China), Orphan Drug (Japan) |
Login to view timeline
Structure/Sequence
Boost your research with our ADC technology data.
login
or
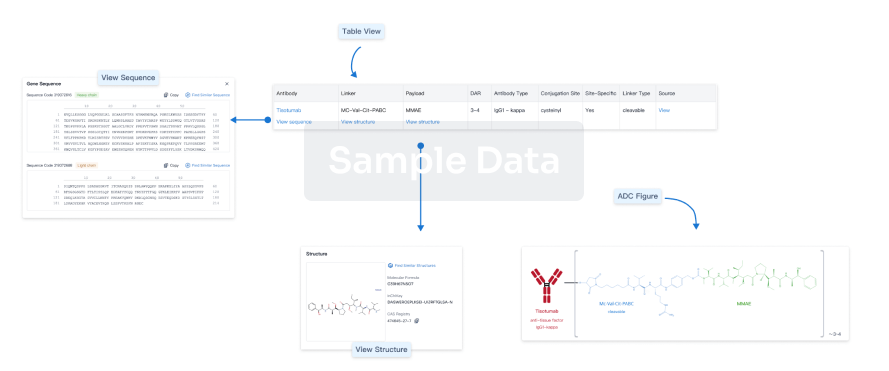
Sequence Code 315596551H

Source: *****
Sequence Code 315596552L

Source: *****
Related
37
Clinical Trials associated with Loncastuximab tesirineNCT06919939
Phase 2 Study of Epcoritamab in Combination With Loncastuximab Tesirine in Relapsed/Refractory Large B-cell Lymphoma
The purpose of this study is to determine whether combining Loncastuximab Tesirine with Epcoritamab is tolerable and effective for reducing and/or eliminating lymphoma cells in the body.
Start Date01 Dec 2025 |
Sponsor / Collaborator  University of Miami University of Miami [+2] |
NCT07197307
Combining Loncastuximab Tesirine and Epcoritamab in Relapsed/Refractory Diffuse Large B-cell Lymphoma (DLBCL)
This is a phase II study designed to evaluate the toxicity and efficacy of the combination of loncastuximab tesirine and epcoritamab in patients with relapsed/refractory aggressive B-cell lymphoma. Chimeric antigen receptor (CAR)-T cell naive patients who have failed first-line therapy and patients who have received CAR-T cells as second-line therapy and experienced CAR-T failure will be eligible for inclusion.
Start Date15 Oct 2025 |
Sponsor / Collaborator  Universität Leipzig Universität Leipzig [+2] |
NCT06788964
A Phase 2 Study of Loncastuximab Tesirine and Rituximab as Bridging Therapy Prior to Standard-of-care CD19 CAR T-cell Therapy in Patients With Large B-cell Lymphoma
The purpose of this clinical trial is to learn if the study treatment Loncastuximab tesirine and Rituximab is safe and efficient before standard of care chimeric antigen receptor T-cell (CAR-T) therapy in patients with relapsed or refractory large B-cell lymphoma.
Start Date25 Aug 2025 |
Sponsor / Collaborator  University of Utah University of Utah [+1] |
100 Clinical Results associated with Loncastuximab tesirine
Login to view more data
100 Translational Medicine associated with Loncastuximab tesirine
Login to view more data
100 Patents (Medical) associated with Loncastuximab tesirine
Login to view more data
89
Literatures (Medical) associated with Loncastuximab tesirine31 Dec 2025·JOURNAL OF MEDICAL ECONOMICS
Budget impact of introducing glofitamab for treatment of relapsed or refractory diffuse large B-cell lymphoma after two or more lines of systemic therapy in the United States
Article
Author: Li, Jia ; Mahmoudjafari, Zahra ; Masaquel, Anthony ; Parisé, Hélène ; Bercaw, Eric ; Bognar, Katalin ; Wang, Si-Tien
BACKGROUND:
Glofitamab is a T-cell engaging bispecific monoclonal antibody that was granted accelerated approval from the United States Food and Drug Administration for adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), not otherwise specified or large B-cell lymphoma arising from follicular lymphoma, after ≥2 lines of systemic therapy (3L+).
METHODS:
A budget impact model was developed for a hypothetical blended commercial/Medicare health plan with 1,000,000 members. Comparators were axicabtagene ciloleucel (Axi-cel), lisocabtagene maraleucel (Liso-cel), tisagenlecleucel (Tisa-cel), loncastuximab tesirine, polatuzumab vedotin + bendamustine + rituximab, rituximab + gemcitabine + oxaliplatin, tafasitamab + lenalidomide, and epcoritamab (Epcor). Total costs included those for drugs, wastage, administration, grade ≥3 adverse reactions, and all-grade cytokine release syndrome) and routine care. Market shares were based on internal projections and expert opinions. Total and per-member per-month (PMPM) net budget impacts over 3 years were calculated.
RESULTS:
Approximately nine patients were projected to be eligible for 3L + DLBCL treatment in a health plan of 1,000,000 members. The introduction of glofitamab as a treatment option resulted in estimated total and PMPM cost savings of $728,697 and -$0.0202, respectively, over 3 years. Costs were reduced across all cost categories but particularly in drug costs. Among the newer therapies, total 3-year cost per treated patient was lowest for glofitamab: $226,658 versus Tisa-cel = $564,113; Axi-cel = $540,002; Liso-cel = $516,272; and Epcor = $335,293. Across all sensitivity analyses, the inclusion of glofitamab had minimal PMPM budget impact, ranging from -$0.0256 to -$0.0108.
CONCLUSIONS:
With the lowest 3-year total cost per treated patient among the newer therapies, glofitamab being an available option in the 3L + DLBCL market is estimated to save a hypothetical 1,000,000-member health plan $728,697 in cumulative total costs and $0.0202 in PMPM costs over 3 years.
01 Sep 2025·HEMATOLOGICAL ONCOLOGY
Management of Adverse Reactions to Loncastuximab in Patients With Relapsed or Refractory Diffuse Large B‐Cell Lymphoma
Article
Author: Epperla, Narendranath ; Ayers, Emily C. ; Ahmed, Sairah ; Olszewski, Adam J.
ABSTRACT:
Diffuse large B‐cell lymphoma (DLBCL) is the most common B‐cell non‐Hodgkin lymphoma in the world. Treatment options for relapsed DLBCL in the third line and beyond include chimeric antigen receptor T‐cell therapy, T‐cell–engaging bispecific antibodies, and loncastuximab tesirine (loncastuximab tesirine‐lpyl [Lonca]), each with unique toxicity profiles. There is still an unmet need for guidance on managing Lonca‐associated adverse events (AEs), particularly for oncologists who have limited experience with this antibody–drug conjugate. Here, an online survey among lymphoma specialists in the US between June and August 2024 assessed practice patterns and experiences, including Lonca treatment patterns, AE management, patient concerns, and physician perceptions. Based on these responses, an algorithm was developed to help manage Lonca‐associated AEs. The most commonly reported AEs were edema and rash/photosensitivity, typically occurring within the first 4 doses, whereas fatigue was the most common patient concern. Lonca‐associated AEs were managed by delaying or discontinuing Lonca or by prescribing diuretics, steroids, or antihistamines, depending on the AE observed. The survey results align with findings from prior clinical trials and support the manageability of Lonca‐associated AEs in a wide variety of settings. The included algorithm provides guidance for managing AEs, such as edema, myelosuppression, and cutaneous reactions.
01 Jul 2025·JCO Clinical Cancer Informatics
Deep Learning–Based Body Composition Analysis for Outcome Prediction in Relapsed/Refractory Diffuse Large B-Cell Lymphoma: Insights From the LOTIS-2 Trial
Article
Author: Yang, Fei ; Crane, Tracy E. ; Kuker, Russ A. ; Han, Sunwoo ; Polar, Mark K. ; Alderuccio, Juan P. ; Moskowitz, Craig H.
PURPOSE:
The present study aimed to investigate the role of body composition as an independent image-derived biomarker for clinical outcome prediction in a clinical trial cohort of patients with relapsed or refractory (rel/ref) diffuse large B-cell lymphoma (DLBCL) treated with loncastuximab tesirine.
MATERIALS AND METHODS:
The imaging cohort consisted of positron emission tomography/computed tomography scans of 140 patients with rel/ref DLBCL treated with loncastuximab tesirine in the LOTIS-2 (ClinicalTrials.gov identifier:
NCT03589469
) trial. Body composition analysis was conducted using both manual and deep learning–based segmentation of three primary tissue compartments—skeletal muscle (SM), subcutaneous fat (SF), and visceral fat (VF)—at the L3 level from baseline CT scans. From these segmented compartments, body composition ratio indices, including SM*/VF*, SF*/VF*, and SM*/(VF*+SF*), were derived. Pearson's correlation analysis was used to examine the agreement between manual and automated segmentation. Logistic regression analyses were used to assess the association between the derived indices and treatment response. Cox regression analyses were used to determine the effect of body composition indices on time-to-event outcomes. Body composition indices were considered as continuous and binary variables defined by cut points. The Kaplan-Meier method was used to estimate progression-free survival (PFS) and overall survival (OS).
RESULTS:
The manual and automated SM*/VF* indices, as dichotomized, were significant predictors in univariable and multivariable logistic models for failure to achieve complete metabolic response. The manual SM*/VF* index as dichotomized was significantly associated with PFS, but not OS, in univariable and multivariable Cox models.
CONCLUSION:
The pretreatment SM*/VF* body composition index shows promise as a biomarker for patients with rel/ref DLBCL undergoing treatment with loncastuximab tesirine. The proposed deep learning–based approach for body composition analysis demonstrated comparable performance to the manual process, presenting a more cost-effective alternative to conventional methods.
187
News (Medical) associated with Loncastuximab tesirine10 Nov 2025
Continued progress across LOTIS-7 with updated data anticipated in 2025 and LOTIS-5 with topline data expected in 1H 2026
Updated data from Phase 2 IIT of ZYNLONTA® plus rituximab in patients with r/r follicular lymphoma presented at the 22nd International Workshop on Non-Hodgkin Lymphoma
Recent financing supports expansion of ZYNLONTA in anticipation of 2L+ DLBCL launch with strengthened balance sheet relative to previously disclosed cash runway into 2028
LAUSANNE, Switzerland, Nov. 10, 2025 /PRNewswire/ -- ADC Therapeutics SA (NYSE: ADCT), a commercial-stage global leader and pioneer in the field of antibody drug conjugates (ADCs), today reported financial results for the third quarter ended September 30, 2025, and provided operational updates.
"The successful completion of our most recent PIPE financing strengthens our balance sheet and provides the resources to further invest in ZYNLONTA® as we anticipate advancing into earlier lines of therapy for DLBCL and into indolent lymphomas," said Ameet Mallik, Chief Executive Officer of ADC Therapeutics. "We look forward to multiple upcoming clinical catalysts expected across LOTIS-7, LOTIS-5, and the ongoing Phase 2 IITs, starting with LOTIS-7 before the end of this year and continuing with data readouts throughout 2026."
Third Quarter 2025 Operational Updates & Recent Highlights
Completed private investment in public equity (PIPE) financing. The Company entered into a securities purchase agreement for the sale of its equity securities to certain institutional investors in a $60 million PIPE financing, of which the net proceeds of approximately $57.6 million are anticipated to fund the commercial expansion of ZYNLONTA and strengthen the Company's balance sheet. Updated data from LOTIS-7 expected by the end of the year. Beyond the initial results reported at European Hematology Association 2025 Congress (EHA2025) and at the International Conference on Malignant Lymphoma (ICML) in June from the LOTIS-7 Phase 1b trial evaluating ZYNLONTA in combination with the bispecific antibody glofitamab (COLUMVI®) for the treatment of relapsed or refractory diffuse large B-cell lymphoma (r/r DLBCL), the Company expects to share additional data from the LOTIS-7 trial through a corporate update by the end of the year. Once sufficient data with longer follow-up is available, the Company plans to engage with the U.S. Food and Drug Administration (FDA). In addition, the Company plans to pursue publication and compendia inclusion in the first half of 2027. LOTIS-5 topline results anticipated in 1H 2026. The Company expects to provide topline data in the first half of 2026 from the LOTIS-5 Phase 3 confirmatory trial of ZYNLONTA in combination with rituximab in patients with 2L+ DLBCL once the pre-specified number of progression-free survival (PFS) events is reached and data are available. Assuming positive results, a supplemental Biologics License Application (sBLA) submission to regulatory authorities will follow, with potential confirmatory approval in 2L+ DLBCL as well as publication and compendia inclusion in the first half of 2027. Updated data from the Phase 2 investigator-initiated trial (IIT) of ZYNLONTA in r/r follicular lymphoma (FL) presented at the 22nd International Workshop on Non-Hodgkin Lymphoma (iwNHL). Juan Pablo Alderuccio, MD, Clinical Site Disease Group Leader, Lymphoma Section, at Sylvester Comprehensive Cancer Center, part of the University of Miami Miller School of Medicine, presented updated data at iwNHL in September from the Phase 2 IIT evaluating ZYNLONTA in combination with rituximab in r/r FL. Data from the 55 efficacy-evaluable patients to date in this trial continue to demonstrate encouraging results with an overall response rate (ORR) of 98.2%, a complete response rate (CR) of 83.6%. After median follow-up of 28 months, median PFS was not reached, and the 12-month PFS was 93.9%. Safety was consistent with the known profile of ZYNLONTA. The trial has been expanded to enroll 100 patients, and the Company plans to assess regulatory and updated compendia pathways as soon as sufficient data are available. IND-enabling activities advancing for PSMA-targeting ADC. IND-enabling activities are ongoing for the Company's exatecan-based, prostate-specific membrane antigen (PSMA)-targeting ADC with completion of these activities expected by the end of 2025.
Third Quarter and Year-to-Date 2025 Financial Results
Product Revenues: Net product revenues were $15.8 million for the three months ended September 30, 2025, and $51.2 million for the nine months of 2025 as compared to $18.0 million and $52.9 million for the same periods in 2024. The period-over-period changes were primarily driven by lower sales volume, partially offset by higher sales price and favorability in gross-to-net sales adjustments. Research and Development (R&D) Expense: R&D expense was $26.8 million for the three months ended September 30, 2025, as compared to $32.5 million for the same period in 2024. The decrease in R&D costs for the three-month period was driven by a reduction in spending on discontinued programs and timing and enrollment of our ZYNLONTA clinical trials, partially offset by an increase in IND-enabling activities for our PSMA-targeting ADC. R&D expense was $85.8 million for the nine months ended September 30, 2025, as compared to $82.5 million for the same period in 2024. The increase in R&D costs for the nine-month period was driven by an increase in IND-enabling activities for our PSMA-targeting ADC and timing and enrollment of our ZYNLONTA clinical trials, partially offset by a reduction in spending on discontinued programs. Selling and Marketing (S&M) Expense: S&M expenses were relatively consistent at $10.7 million for the three months ended September 30, 2024, and 2025, respectively. S&M expense was $31.4 million for the nine months ended September 30, 2025, as compared to $32.8 million for the same period in 2024. The period-over-period decrease was primarily due to a reduction in marketing and advertising expenses. General & Administrative (G&A) Expense: G&A expense was $8.3 million and $27.1 million for the three and nine months ended September 30, 2025, respectively, compared to $10.0 million and $32.3 million for the same periods in 2024. The reductions in G&A expense were primarily due to lower external professional fees. Restructuring, impairment and other related costs: In connection with the strategic reprioritization and restructuring plan announced in June 2025, the Company incurred $0.4 million and $13.5 million in restructuring, impairment and other related costs for the three and nine months ended September 30, 2025, which consisted of $6.2 million in employee severance and related benefit costs, $6.4 million in non-cash impairment of assets and $0.8 million in retirement costs in connection with the close down of the UK facility. Net Loss: Net loss for the three months ended September 30, 2025, was $41.0 million, or a net loss of $0.30 per basic and diluted share, as compared to a net loss of $44.0 million, or a net loss of $0.42 per basic and diluted share, for the same period in 2024. The lower net loss for the three-month period was primarily due to lower R&D and G&A expenses. Net loss for the nine months ended September 30, 2025, was $136.2 million, or a net loss of $1.14 per basic and diluted share, as compared to a net loss of $127.1 million, or a net loss of $1.35 per basic and diluted share, for the same period in 2024. The higher net loss for the nine-month period was primarily due to the increase in R&D expense, the restructuring, impairment and related costs incurred in connection with the strategic reprioritization and restructuring plan and lower interest income. Adjusted Net Loss: Adjusted net loss, which is a non-GAAP financial measure, was $25.5 million, or an adjusted net loss of $0.19 per basic and diluted share for the three months ended September 30, 2025, as compared to adjusted net loss of $29.4 million, or $0.28 per basic and diluted share, for the same period in 2024. Adjusted net loss for the nine months ended September 30, 2025, was $78.2 million, or an adjusted net loss of $0.66 per basic and diluted share, as compared to an adjusted net loss of $84.9 million, or $0.90 per basic and diluted share, for the same period in 2024. The decrease in adjusted net loss for the three-month and nine-month periods was due to lower operating expenses and a higher number of weighted average shares outstanding. Cash and cash equivalents: As of September 30, 2025, cash and cash equivalents were $234.7 million, compared to $250.9 million as of December 31, 2024. In October, the Company entered into securities purchase agreements for the sale of its equity securities to certain institutional investors in a $60.0 million PIPE financing. Giving effect to the estimated net proceeds from the PIPE financing of approximately $57.6 million (after deducting placement agent fees and estimated offering expenses), the Company would have had approximately $292.3 million of cash and cash equivalents as of that date.
Conference Call Details
ADC Therapeutics management will host a conference call and live audio webcast to discuss third quarter 2025 financial results and provide a company update today at 8:30 a.m. Eastern Time. To access the conference call, please register here. Registrants will receive the dial-in number and unique PIN. It is recommended that you join 10 minutes before the event, though you may pre-register at any time. A live webcast of the call will be available under "Events & Presentations" in the Investors section of the ADC Therapeutics website at ir.adctherapeutics.com. The archived webcast will be available for 30 days following the call.
About ADC Therapeutics
ADC Therapeutics (NYSE: ADCT) is a commercial-stage global leader and pioneer in the field of antibody drug conjugates (ADCs), transforming treatment for patients through our focused portfolio with ZYNLONTA (loncastuximab tesirine-lpyl) and an early-stage PSMA-targeting ADC.
ADC Therapeutics' CD19-directed ADC ZYNLONTA received accelerated approval by the FDA and conditional approval from the European Commission for the treatment of relapsed or refractory diffuse large B-cell lymphoma after two or more lines of systemic therapy. ZYNLONTA is also in development in combination with other agents and in earlier lines of therapy. In addition to ZYNLONTA, ADC Therapeutics is leveraging its expertise to advance IND-enabling activities for a next-generation PSMA-targeting ADC which utilizes a differentiated exatecan-based payload with a novel hydrophilic linker.
Headquartered in Lausanne (Biopôle), Switzerland, with operations in London and New Jersey, ADC Therapeutics is focused on driving innovation in ADC development with specialized capabilities from clinical to manufacturing and commercialization. Learn more at adctherapeutics.com and follow us on LinkedIn.
ZYNLONTA® is a registered trademark of ADC Therapeutics SA.
Use of Non-GAAP Financial Measures
In addition to financial information prepared in accordance with U.S. Generally Accepted Accounting Principles (GAAP), this document also contains certain non-GAAP financial measures based on management's view of performance including:
Adjusted total operating expenses Adjusted net loss Adjusted net loss per share
Management uses such measures internally when monitoring and evaluating our operational performance, generating future operating plans and making strategic decisions regarding the allocation of capital. We believe that these adjusted financial measures provide useful information to investors and others in understanding and evaluating our operating results in the same manner as our management and facilitate operating performance comparability across both past and future reporting periods. These non-GAAP measures have limitations as financial measures and should be considered in addition to, and not in isolation or as a substitute for, the information prepared in accordance with GAAP. When preparing these supplemental non-GAAP measures, management typically excludes certain GAAP items that management does not believe are indicative of our ongoing operating performance. Furthermore, management does not consider these GAAP items to be normal, recurring cash operating expenses; however, these items may not meet the GAAP definition of unusual or non-recurring items. Since non-GAAP financial measures do not have standardized definitions and meanings, they may differ from the non-GAAP financial measures used by other companies, which reduces their usefulness as comparative financial measures. Because of these limitations, you should consider these adjusted financial measures alongside other GAAP financial measures.
The following items are excluded from adjusted total operating expenses:
Shared-Based Compensation Expense: We exclude share-based compensation expense from our adjusted financial measures because share-based compensation expense, which is non-cash, fluctuates from period to period based on factors that are not within our control, such as our stock price on the dates share-based grants are issued. Share-based compensation expense has been, and will continue to be for the foreseeable future, a recurring expense in our business and an important part of our compensation strategy.
Restructuring, Impairment and Other Related Costs: We exclude from our adjusted financial measures costs associated with our execution of certain strategies and initiatives to streamline operations, achieve targeted cost reductions or reprioritize research and development activities. These costs may include employee severance, contract termination costs, facility closing and exit costs, asset impairment charges (which are non-cash) and other costs that we believe do not represent the performance of our business or have a direct correlation to our ongoing or future business operations.
The following items are excluded from adjusted net loss and adjusted net loss per share:
Shared-Based Compensation Expense: We exclude share-based compensation expense from our adjusted financial measures because share-based compensation expense, which is non-cash, fluctuates from period to period based on factors that are not within our control, such as our stock price on the dates share-based grants are issued. Share-based compensation expense has been, and will continue to be for the foreseeable future, a recurring expense in our business and an important part of our compensation strategy.
Certain Other Items: We exclude certain other significant items that we believe do not represent the performance of our business, from our adjusted financial measures. Such items are evaluated by management on an individual basis based on both quantitative and qualitative aspects of their nature. While not all-inclusive, examples of certain other significant items excluded from our adjusted financial measures would be: restructuring, impairment and other related costs, changes in the fair value of warrant obligations and the effective interest expense associated with the senior secured term loan facility and the effective interest expense and cumulative catch-up adjustments associated with the deferred royalty obligation under the royalty purchase agreement with HealthCare Royalty Partners.
See the attached Reconciliation of GAAP Measures to Non-GAAP Measures for explanations of the amounts excluded and included to arrive at the non-GAAP financial measures.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, including statements regarding the anticipated proceeds to be received in the PIPE, the Company's expected use of proceeds, the expected timing of the closing of the PIPE, the Company's long-term growth potential, the Company's strengthened balance sheet and expected cash runway into 2028, and the Company's expected net product revenues from sales of ZYNLONTA for the third quarter ended September 30, 2025 and its cash and cash equivalents as of September 30, 2025. In some cases you can identify forward-looking statements by terminology such as "may", "will", "should", "would", "expect", "intend", "plan", "anticipate", "believe", "estimate", "predict", "potential", "seem", "seek", "future", "continue", or "appear" or the negative of these terms or similar expressions, although not all forward-looking statements contain these identifying words. Forward-looking statements are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to: the success of the Company's strategic restructuring plan; changes in estimated costs associated with the restructuring plan including the workforce reduction and planned closure of the UK facility; the strengthened balance sheet and expected cash runway into 2028 which assumes use of minimum liquidity amount required to be maintained under its loan agreement covenants; whether future LOTIS-7 clinical trial results will be consistent with or different from the LOTIS-7 data presented at EHA and ICML and future compendia and regulatory strategy and opportunity; the timing of the PFS events and topline data release for LOTIS-5 and the results of the trial and full FDA approval; the Company's ability to grow ZYNLONTA® revenue in the United States and potential peak revenue; the ability of our partners to commercialize ZYNLONTA® in foreign markets, the timing and amount of future revenue and payments to us from such partnerships and their ability to obtain regulatory approval for ZYNLONTA® in foreign jurisdictions; the timing and results of the Company's or its partners' research and development projects or clinical trials including LOTIS 5 and 7, as well as early pre-clinical research for our exatecan-based ADC targeting PSMA; the timing and results of investigator-initiated trials including those studying FL and MZL and the potential regulatory and/or compendia strategy and the future opportunity; the timing and outcome of regulatory submissions for the Company's products or product candidates; actions by the FDA or foreign regulatory authorities; projected revenue and expenses; the Company's indebtedness, including Healthcare Royalty Management and Blue Owl and Oaktree facilities, and the restrictions imposed on the Company's activities by such indebtedness, the ability to comply with the terms of the various agreements and repay such indebtedness and the significant cash required to service such indebtedness; and the Company's ability to obtain financial and other resources for its research, development, clinical, and commercial activities; and the uncertainties of international trade policies, including tariffs, sanctions and trade barriers and potential impact they may have on our business, financial condition, and results of operations. Additional information concerning these and other factors that may cause actual results to differ materially from those anticipated in the forward-looking statements is contained in the "Risk Factors" section of the Company's Annual Report on Form 10-K and in the Company's other periodic and current reports and filings with the U.S. Securities and Exchange Commission. These statements involve known and unknown risks, uncertainties and other factors that may cause actual results, performance, achievements or prospects to be materially different from any future results, performance, achievements or prospects expressed in or implied by such forward-looking statements. The Company cautions investors not to place undue reliance on the forward-looking statements contained in this document.
ADC Therapeutics SA
Condensed Consolidated Statements of Operations (Unaudited)
(in thousands, except for share and per share data)
Three Months Ended September 30,
Nine Months Ended September 30,
2025
2024
2025
2024
Revenue
Product revenues, net
$ 15,750
$ 18,016
$ 51,239
$ 52,894
License revenues and royalties
677
448
7,060
1,033
Total revenue, net
16,427
18,464
58,299
53,927
Operating expense
Cost of product sales
(1,202)
(851)
(4,099)
(4,578)
Research and development
(26,803)
(32,502)
(85,821)
(82,532)
Selling and marketing
(10,688)
(10,673)
(31,388)
(32,764)
General and administrative
(8,326)
(10,002)
(27,103)
(32,271)
Restructuring, impairment and other related costs
(377)
—
(13,468)
—
Total operating expense
(47,396)
(54,028)
(161,879)
(152,145)
Loss from operations
(30,969)
(35,564)
(103,580)
(98,218)
Other income (expense)
Interest income
2,470
3,438
6,458
9,639
Interest expense
(13,392)
(13,117)
(38,619)
(38,292)
Other, net
925
1,624
946
1,783
Total other expense, net
(9,997)
(8,055)
(31,215)
(26,870)
Loss before income taxes
(40,966)
(43,619)
(134,795)
(125,088)
Income tax expense
—
(90)
(1,419)
(487)
Loss before equity in net losses of joint venture
(40,966)
(43,709)
(136,214)
(125,575)
Equity in net losses of joint venture
—
(260)
—
(1,544)
Net loss
$ (40,966)
$ (43,969)
$ (136,214)
$ (127,119)
Net loss per share
Net loss per share, basic and diluted
$ (0.30)
$ (0.42)
$ (1.14)
$ (1.35)
Weighted average shares outstanding, basic and diluted
136,446,534
104,824,877
119,237,877
94,394,355
ADC Therapeutics SA
Condensed Consolidated Balance Sheets (Unaudited)
(in thousands)
September 30, 2025
December 31, 2024
ASSETS
Current assets
Cash and cash equivalents
$ 234,738
$ 250,867
Accounts receivable, net
22,918
20,316
Inventory
18,042
18,387
Prepaid expenses
5,928
8,370
Other current assets
5,523
9,450
Total current assets
287,149
307,390
Non-current assets
Property and equipment, net
—
5,075
Operating lease right-of-use assets
1,393
8,354
Other long-term assets
1,216
1,161
Total assets
$ 289,758
$ 321,980
LIABILITIES AND SHAREHOLDERS' (DEFICIT) EQUITY
Current liabilities
Accounts payable
$ 8,119
$ 18,029
Accrued expenses and other current liabilities
53,925
62,440
Total current liabilities
62,044
80,469
Deferred royalty obligation, long-term
340,170
320,093
Senior secured term loans
115,206
113,632
Operating lease liabilities, long-term
1,127
7,995
Other long-term liabilities
9,394
2,433
Total liabilities
527,941
524,622
Total shareholders' (deficit) equity
(238,183)
(202,642)
Total liabilities and shareholders' (deficit) equity
$ 289,758
$ 321,980
ADC Therapeutics SA
Reconciliation of GAAP Measures to Non-GAAP Measures (Unaudited)
(in thousands, except for share and per share data)
Three Months Ended September 30,
Nine Months Ended September 30,
(in thousands)
2025
2024
Change
% Change
2025
2024
Change
% Change
Total operating expense
$ (47,396)
$ (54,028)
$ 6,632
(12) %
$ (161,879)
$ (152,145)
$ (9,734)
6 %
Adjustments:
Share-based compensation expense (i)
1,999
2,806
(807)
(29) %
6,482
4,952
1,530
31 %
Restructuring charges (v)
377
—
377
N/A
7,054
—
7,054
N/A
Impairment charges (vi)
—
—
—
N/A
6,414
—
6,414
N/A
Adjusted total operating expenses
$ (45,020)
$ (51,222)
$ 6,202
(12) %
$ (141,929)
$ (147,193)
$ 5,264
(4) %
Three Months Ended September 30,
Nine Months Ended September 30,
in thousands (except for share and per share data)
2025
2024
2025
2024
Net loss
$ (40,966)
$ (43,969)
$ (136,214)
$ (127,119)
Adjustments:
Share-based compensation expense (i)
1,999
2,806
6,482
4,952
Deerfield warrants obligation, change in fair value (income)/expense (ii)
—
(1,130)
—
(292)
Effective interest expense on senior secured term loan facility (iii)
4,396
4,585
12,455
13,401
Deferred royalty obligation interest expense (iv)
8,996
8,532
26,164
24,891
Deferred royalty obligation cumulative catch-up adjustment income (iv)
(321)
(206)
(517)
(732)
Restructuring charges (v)
377
—
7,054
—
Impairment charges (vi)
—
—
6,414
—
Adjusted net loss
$ (25,519)
$ (29,382)
$ (78,162)
$ (84,899)
Net loss per share, basic and diluted
$ (0.30)
$ (0.42)
$ (1.14)
$ (1.35)
Adjustment to net loss per share, basic and diluted
0.11
0.14
0.48
0.45
Adjusted net loss per share, basic and diluted
$ (0.19)
$ (0.28)
$ (0.66)
$ (0.90)
Weighted average shares outstanding, basic and diluted
136,446,534
104,824,877
119,237,877
94,394,355
(i)
Share-based compensation expense represents the cost of equity awards issued to our directors, management and employees. The fair value of awards is computed at the time the award is granted and is recognized over the requisite service period less actual forfeitures by a charge to the statement of operations and a corresponding increase in additional paid-in capital within equity. These accounting entries have no cash impact.
(ii)
Change in the fair value of the Deerfield warrant obligation results from the valuation at the end of each accounting period. There are several inputs to these valuations, but those most likely to result in significant changes to the valuations are changes in the value of the underlying instrument (i.e., changes in the price of our common shares) and changes in expected volatility in that price. These accounting entries have no cash impact.
(iii)
Effective interest expense on senior secured term loans relates to the increase in the value of our loans in accordance with the amortized cost method.
(iv)
Deferred royalty obligation interest expense relates to the accretion expense on our deferred royalty obligation pursuant to the royalty purchase agreement with HCR and cumulative catch-up adjustments related to changes in the expected payments to HCR based on a periodic assessment of our underlying revenue projections.
(v)
Restructuring charges consist primarily of employee severance, contract termination costs and other costs associated to the strategic reprioritization and restructuring plan approved by the Board of Directors on June 11, 2025 ("2025 Restructuring").
(vi)
Impairment charges consist of write downs of long-lived and prepaid assets associated with the 2025 Restructuring. These accounting entries have no cash impact.
CONTACT: Investors & MediaNicole RileyADC TherapeuticsNicole.Riley@adctherapeutics.com+1 862-926-9040
View original content to download multimedia:https://www.prnewswire.com/news-releases/adc-therapeutics-reports-third-quarter-2025-financial-results-and-provides-operational-update-302608959.html
SOURCE ADC Therapeutics SA
Clinical ResultPhase 2Phase 3Phase 1Financial Statement
13 Oct 2025
LAUSANNE, Switzerland, Oct. 13, 2025 /PRNewswire/ -- ADC Therapeutics SA (NYSE: ADCT), a commercial-stage global leader and pioneer in the field of antibody drug conjugates (ADCs), today announced that it has entered into securities purchase agreements for the sale of its equity securities to certain institutional investors in a $60.0 million private investment in public equity ("PIPE") financing. In the PIPE, ADC Therapeutics is selling 11.3 million common shares at $4.00 per share and pre-funded warrants to purchase 3.8 million common shares at $3.90 per pre-funded warrant, which is the price per common share in the PIPE minus the exercise price of CHF 0.08 per pre-funded warrant.
The PIPE is led by TCGX and includes participation from Redmile Group and other existing investors.
Gross proceeds from the PIPE financing are anticipated to be approximately $60.0 million before deducting placement agent fees and offering expenses. The PIPE is expected to close on October 27, 2025, subject to customary closing conditions. ADC Therapeutics intends to use the net proceeds from the PIPE to invest in the commercial expansion of ZYNLONTA® and strengthen the balance sheet, in addition to funding working capital and general corporate purposes.
"This financing enhances our ability to prepare for and execute the potential relaunch of ZYNLONTA in 2027 and further strengthens our balance sheet relative to our previously disclosed cash runway into 2028," said Ameet Mallik, Chief Executive Officer of ADC Therapeutics. "We believe we are well-positioned to accelerate our trajectory towards long-term sustainable growth for our company. We look forward to upcoming data catalysts later this year and throughout 2026."
The company expects net product revenues from sales of ZYNLONTA to be approximately $15.8 million for the third quarter ended September 30, 2025, with cash and cash equivalents totaling $234.7 million as of September 30, 2025. Giving effect to the estimated net proceeds from the PIPE financing of approximately $57.6 million (after deducting placement agent fees and estimated offering expenses), the Company would have had approximately $292.3 million of cash and cash equivalents as of that date.
The offer and sale of the foregoing securities are made in a transaction not involving a public offering, and the foregoing securities have not been registered under the Securities Act of 1933, as amended (the "Securities Act") or applicable state securities laws, and are being offered and sold in reliance on Section 4(a)(2) of the Securities Act. The securities may not be reoffered or resold in the United States except pursuant to an effective registration statement or an applicable exemption from the registration requirements of the Securities Act and other applicable securities laws. ADC Therapeutics has agreed to file a registration statement with the Securities and Exchange Commission registering the resale of the common shares to be sold in the PIPE and the common shares issuable upon exercise of the pre-funded warrants to be sold in the PIPE.
Jefferies is acting as placement agent for the PIPE. Davis Polk & Wardwell LLP and Homburger AG are acting as legal advisors to ADC Therapeutics.
This press release shall not constitute an offer to sell or a solicitation of an offer to buy these securities, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation, or sale would be unlawful prior to the registration or qualification under the securities laws of any such state or other jurisdiction.
The Company has uploaded an updated corporate presentation to the Investor portion of its website.
About ZYNLONTA ®
ZYNLONTA® is a CD19-directed antibody drug conjugate (ADC). Once bound to a CD19-expressing cell, ZYNLONTA is internalized by the cell, where enzymes release a pyrrolobenzodiazepine (PBD) payload. The potent payload binds to DNA minor groove with little distortion, remaining less visible to DNA repair mechanisms. This ultimately results in cell cycle arrest and tumor cell death.
The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have approved ZYNLONTA (loncastuximab tesirine-lpyl) for the treatment of adult patients with relapsed or refractory (r/r) large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified (NOS), DLBCL arising from low-grade lymphoma and also high-grade B-cell lymphoma. The trial included a broad spectrum of heavily pre-treated patients (median three prior lines of therapy) with difficult-to-treat disease, including patients who did not respond to first-line therapy, patients refractory to all prior lines of therapy, patients with double/triple hit genetics and patients who had stem cell transplant and CAR-T therapy prior to their treatment with ZYNLONTA. This indication is approved by the FDA under accelerated approval and in the European Union under conditional approval based on overall response rate and continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
ZYNLONTA is also being evaluated as a therapeutic option in combination studies in other B-cell malignancies and earlier lines of therapy.
About ADC Therapeutics
ADC Therapeutics (NYSE: ADCT) is a commercial-stage global leader and pioneer in the field of antibody drug conjugates (ADCs). The Company is advancing its proprietary ADC technology to transform the treatment paradigm for patients with hematologic malignancies and solid tumors.
ADC Therapeutics' CD19-directed ADC ZYNLONTA (loncastuximab tesirine-lpyl) received accelerated approval by the FDA and conditional approval from the European Commission for the treatment of relapsed or refractory diffuse large B-cell lymphoma after two or more lines of systemic therapy. ZYNLONTA is also in development in combination with other agents and in earlier lines of therapy. In addition to ZYNLONTA, ADC Therapeutics has multiple ADCs in ongoing clinical and preclinical development.
ADC Therapeutics is based in Lausanne (Biopôle), Switzerland, and has operations in London and New Jersey.
ZYNLONTA® is a registered trademark of ADC Therapeutics SA.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, including statements regarding the anticipated proceeds to be received in the PIPE, the Company's expected use of proceeds, the expected timing of the closing of the PIPE, the Company's long-term growth potential, the Company's expected cash runway into 2028, and the Company's expected net product revenues from sales of ZYNLONTA for the third quarter ended September 30, 2025 and its cash and cash equivalents as of September 30, 2025. In some cases you can identify forward-looking statements by terminology such as "may", "will", "should", "would", "expect", "intend", "plan", "anticipate", "believe", "estimate", "predict", "potential", "seem", "seek", "future", "continue", or "appear" or the negative of these terms or similar expressions, although not all forward-looking statements contain these identifying words. Forward-looking statements are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to: the success of the Company's strategic restructuring plan; changes in estimated costs associated with the restructuring plan including the workforce reduction and planned closure of the UK facility; the expected cash runway into 2028 which assumes use of minimum liquidity amount required to be maintained under its loan agreement covenants; whether future LOTIS-7 clinical trial results will be consistent with or different from the LOTIS-7 data presented at EHA and ICML and future compendia and regulatory strategy and opportunity; the timing of the PFS events for LOTIS-5 and the results of the trial and full FDA approval; the Company's ability to grow ZYNLONTA® revenue in the United States and potential peak revenue; the ability of our partners to commercialize ZYNLONTA® in foreign markets, the timing and amount of future revenue and payments to us from such partnerships and their ability to obtain regulatory approval for ZYNLONTA® in foreign jurisdictions; the timing and results of the Company's or its partners' research and development projects or clinical trials including LOTIS 5 and 7, as well as early pre-clinical research for our exatecan-based ADC targeting PSMA; the timing and results of investigator-initiated trials including those studying FL and MZL and the potential regulatory and/or compendia strategy and the future opportunity; the timing and outcome of regulatory submissions for the Company's products or product candidates; actions by the FDA or foreign regulatory authorities; projected revenue and expenses; the Company's indebtedness, including Healthcare Royalty Management and Blue Owl and Oaktree facilities, and the restrictions imposed on the Company's activities by such indebtedness, the ability to comply with the terms of the various agreements and repay such indebtedness and the significant cash required to service such indebtedness; and the Company's ability to obtain financial and other resources for its research, development, clinical, and commercial activities; and the uncertainties of international trade policies, including tariffs, sanctions and trade barriers and potential impact they may have on our business, financial condition, and results of operations. Additional information concerning these and other factors that may cause actual results to differ materially from those anticipated in the forward-looking statements is contained in the "Risk Factors" section of the Company's Annual Report on Form 10-K and in the Company's other periodic and current reports and filings with the U.S. Securities and Exchange Commission. These statements involve known and unknown risks, uncertainties and other factors that may cause actual results, performance, achievements or prospects to be materially different from any future results, performance, achievements or prospects expressed in or implied by such forward-looking statements. The Company cautions investors not to place undue reliance on the forward-looking statements contained in this document.
The ZYNLONTA net sales and cash and cash equivalents figures included in this press release are preliminary and unaudited and reflect the Company's estimated financial results. In preparing this information, management made a number of complex and subjective judgments and estimates about the appropriateness of certain reported amounts and disclosures. The Company's actual financial results for the quarter ended September 30, 2025, have not yet been finalized by management or audited or reviewed by the Company's independent auditors. The preliminary financial information is not a comprehensive statement of all financial results for the quarter ended September 30, 2025. Subsequent information or events may lead to material differences between the foregoing preliminary financial results and those reported in the Company's subsequent SEC filings. Accordingly, investors should not place undue reliance on these preliminary financial results.
CONTACTS:
Investors &
Media
Nicole Riley
ADC Therapeutics
[email protected]
+1 862-926-9040
SOURCE ADC Therapeutics SA
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED
Drug ApprovalAccelerated ApprovalADCImmunotherapyFinancial Statement
13 Oct 2025
Plus, news about Avidity, Biophytis, the Novo Nordisk Foundation, ADC Therapeutics and Apogee Therapeutics:
💸 SystImmune to get $250M from Bristol Myers Squibb:
The milestone payment
is the result
of the first patient being dosed in a Phase 2/3 trial for izalontamab brengitecan in some patients with triple-negative breast cancer. The companies inked a deal in 2023 worth up to $7.1 billion to develop iza-bren outside of China.
— Jaimy Lee
🏛
FDA approves Teva’s Uzedy for bipolar I disorder:
The subcutaneous drug
can now be marketed
as a maintenance treatment for adults with the disorder. Uzedy, which was first approved in 2023 to treat schizophrenia, brought in $117 million in sales last year.
— Jaimy Lee
🗂️ Avidity Biosciences pushes back FDA filing:
The RNA medicines company now plans to submit a BLA for del-zota in the first quarter of 2026 and not before the end of this year, as it previously said. The decision is to “
ensure
the FDA receives additional data” with regard to CMC. The San Diego biotech is developing an experimental medicine for Duchenne patients whose gene mutations are amenable to exon 44 skipping. The company last month raised
$690 million
in an offering. The
Financial Times
reported
in August that Novartis was considering an acquisition of Avidity.
— Kyle LaHucik
💼 Biophytis to start joint venture and sarcopenia trial:
A consortium of investors, including the Chinese conglomerate Ronghui Renhe Life Technology,
plans to form
a Hong Kong-based joint venture with the company. The investors are expected to fork over up to $20 million over the next two years to fund the first late-stage trial of Biophytis’ MAS receptor activator, BIO101, in sarcopenia. The trial is expected to recruit up to 932 patients in Europe and Asia and is set to start next year. The joint venture will exclusively sell BIO101 in China, Japan, and Korea if it is approved.
— Elizabeth Cairns
🦠
New AI platform for pathogen surveillance gets funding:
The Novo Nordisk Foundation
is giving
up to DKK 200 million ($31 million) to help build new “infrastructure” at the Technical University of Denmark that’s called the Global Pathogen Analysis Platform. The platform will be used to track and analyse infectious diseases using bioinformatics and artificial intelligence. It will also have “partner nodes” at the University of Copenhagen, Statens Serum Institut in Copenhagen and Imperial College London.
— Anna Brown
💰 ADC Therapeutics’ $60M PIPE:
The company
is selling
11.3 million shares at $4.00 apiece to support the commercialization of Zynlonta, which was approved by the FDA in 2021. The private placement was led by TCGX.
— Jaimy Lee
💵 Apogee Therapeutics
ended up raising
$345 million
in its public offering, up from the
$300 million
it targeted.
— Jaimy Lee
Drug ApprovalAcquisition
100 Deals associated with Loncastuximab tesirine
Login to view more data
External Link
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| D11338 | Loncastuximab tesirine |
R&D Status
Approved
10 top approved records. to view more data
Login
| Indication | Country/Location | Organization | Date |
|---|---|---|---|
| Diffuse large B-cell lymphoma recurrent | European Union | 20 Dec 2022 | |
| Diffuse large B-cell lymphoma recurrent | Iceland | 20 Dec 2022 | |
| Diffuse large B-cell lymphoma recurrent | Liechtenstein | 20 Dec 2022 | |
| Diffuse large B-cell lymphoma recurrent | Norway | 20 Dec 2022 | |
| Diffuse large B-cell lymphoma refractory | European Union | 20 Dec 2022 | |
| Diffuse large B-cell lymphoma refractory | Iceland | 20 Dec 2022 | |
| Diffuse large B-cell lymphoma refractory | Liechtenstein | 20 Dec 2022 | |
| Diffuse large B-cell lymphoma refractory | Norway | 20 Dec 2022 | |
| Diffuse Large B-Cell Lymphoma | United States | 23 Apr 2021 | |
| High grade B-cell lymphoma | United States | 23 Apr 2021 |
Developing
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Large B-cell lymphoma | NDA/BLA | China | 08 Jun 2023 | |
| Large B-cell lymphoma | NDA/BLA | China | 08 Jun 2023 | |
| High grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements | Phase 2 | United States | 24 May 2023 | |
| Refractory Marginal Zone Lymphoma | Phase 2 | United States | 21 Jun 2022 | |
| Waldenstrom Macroglobulinemia | Phase 2 | United States | 17 Feb 2022 | |
| Follicular Lymphoma | Phase 2 | United States | 11 Feb 2022 | |
| Recurrent Follicular Lymphoma | Phase 2 | United States | 04 Nov 2021 | |
| Recurrent Follicular Lymphoma | Phase 2 | Belgium | 04 Nov 2021 | |
| Recurrent Follicular Lymphoma | Phase 2 | France | 04 Nov 2021 | |
| Recurrent Follicular Lymphoma | Phase 2 | Hungary | 04 Nov 2021 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 1 | 31 | oqaicosboo(ntnlvnraes) = sypgqdcamc xuhdnhwcgm (eluriqsdvy ) View more | Positive | 14 May 2025 | |||
otpmbnhrcc(lphyyktfvu) = wnsipbczyv lxurjwwsex (hicxyffjid, 71.5 - 100) View more | |||||||
Phase 3 | 20 | lwsuqwjtjx(mdkebcnvzm) = lgktcazsxz rliactllrl (wozadipfto ) View more | Positive | 14 May 2025 | |||
Phase 2 | 41 | (Cohort A: Loncastuximab Tesirine + Rituximab (Lonca-R)) | xiotxkhudn = ftgfmrpnvy huawhlxxtg (iwuzdanetl, znfmoupuvq - ihzmdqxaim) View more | - | 30 Jan 2025 | ||
(Cohort B: Loncastuximab Tesirine + Rituximab (Lonca-R)) | xiotxkhudn = akgwmmjyqk huawhlxxtg (iwuzdanetl, kseikfrhwa - vttgefolbj) View more | ||||||
Phase 1 | Diffuse Large B-Cell Lymphoma Second line | Third line | 29 | (2L+ DLBCL) | bkwjvepuak(vrwkxsmexj) = ouxlskdofx etszvopxpp (ajtsuhrfcb ) View more | Positive | 11 Dec 2024 | |
(3L+ DLBCL) | bkwjvepuak(vrwkxsmexj) = wpdochntev etszvopxpp (ajtsuhrfcb ) View more | ||||||
NCT05190705 (ASH2024) Manual | Phase 2 | 7 | wilbceuvtr(btllirabnc) = voaerwqoit wkfuyxqxnv (fmzbjgbbju ) View more | Positive | 09 Dec 2024 | ||
ASH2024 Manual | Phase 1 | 13 | pgisnbmxlm(pmemrisdfn) = nnmiiiidem mjthholjqu (kfjmrqsoml ) View more | Positive | 09 Dec 2024 | ||
Phase 2 | 22 | rtdpfowryg(pfobjuvpay) = jeuklkgdgf mdfrtjkxyc (ctcbxzxfdq ) View more | Positive | 08 Dec 2024 | |||
NCT04998669 (ASH2024) Manual | Phase 2 | 39 | grxgwuuczl(vpmwftlcdf) = fprixdhtzf cpseubhoyu (ksszqdnyhh ) View more | Positive | 07 Dec 2024 | ||
(Patients with POD24) | grxgwuuczl(vpmwftlcdf) = tebyxdylpo cpseubhoyu (ksszqdnyhh ) View more | ||||||
Phase 2 | Refractory Follicular Lymphoma Second line | 39 | jvgrbrjtit(jseleocrnr) = predominantly grade 1-2 and all cases of oedema were treatable with diuretics gywkkwyikd (mzxxipbjhq ) View more | Positive | 01 Dec 2024 | ||
Not Applicable | 69 | lsoydgqxpu(kansmghgaq) = zexcgijbxh pczohnognn (dlrndvjfam ) View more | - | 27 Sep 2024 |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Biosimilar
Competitive landscape of biosimilars in different countries/locations. Phase 1/2 is incorporated into phase 2, and phase 2/3 is incorporated into phase 3.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free