Request Demo
Last update 22 Nov 2025
BMS-986263
Last update 22 Nov 2025
Overview
Basic Info
Drug Type siRNA |
Synonyms BMS 986263, ND-L02-S0201 |
Target |
Action inhibitors |
Mechanism SERPINH1 gene inhibitors |
Therapeutic Areas |
Active Indication- |
Inactive Indication |
Originator Organization |
Active Organization- |
Inactive Organization |
License Organization |
Drug Highest PhaseDiscontinuedPhase 2 |
First Approval Date- |
Regulation- |
Login to view timeline
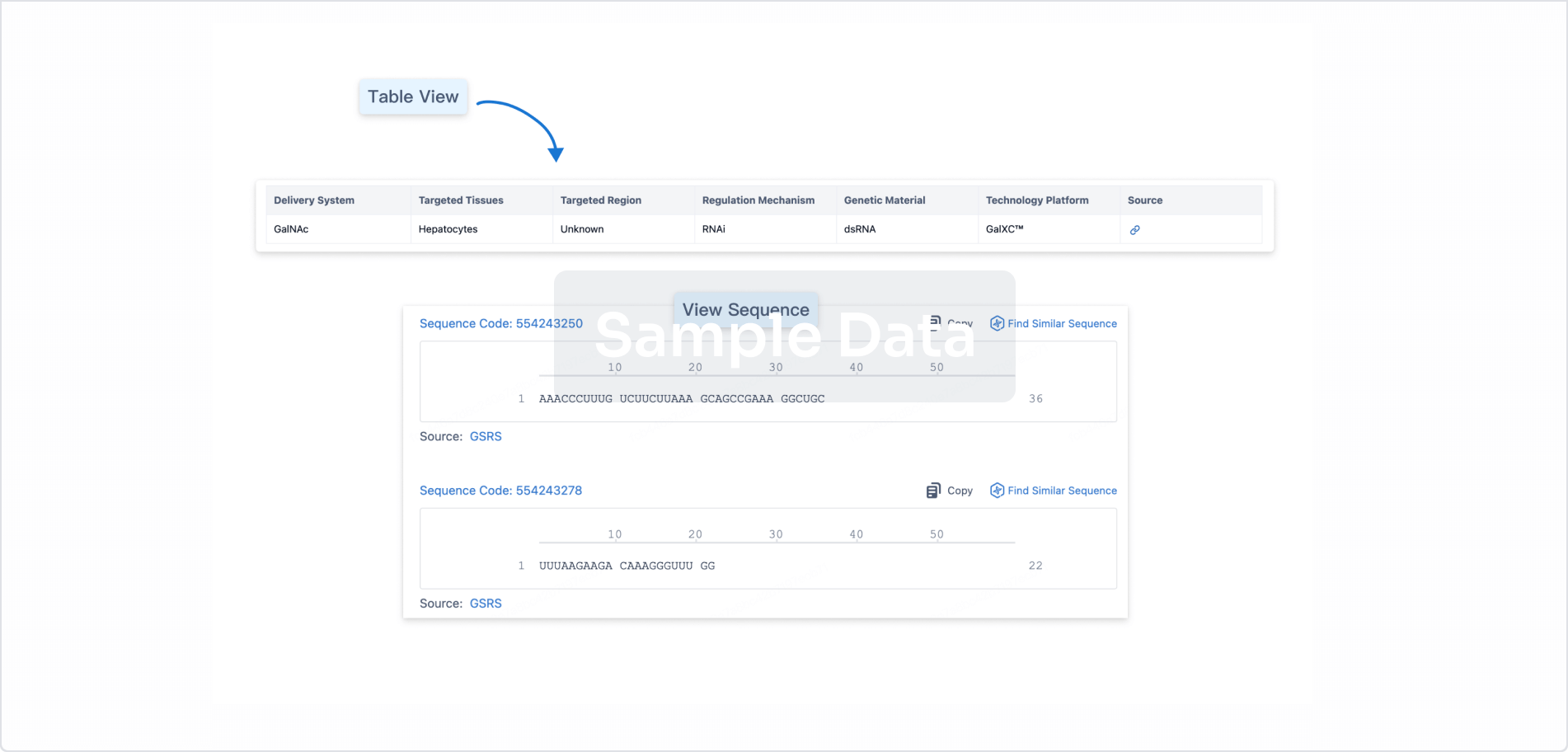
Structure/Sequence
Boost your research with our RNA technology data.
login
or
Related
9
Clinical Trials associated with BMS-986263NCT04267393
A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Multiple-Dose Phase 2 Study to Evaluate the Efficacy and Safety of BMS-986263 in Adults With Compensated Cirrhosis From Nonalcoholic Steatohepatitis (NASH)
The purpose of this randomized study is to assess safety and effectiveness of BMS-986263 in adults with compensated cirrhosis (chronic liver disease) from nonalcoholic steatohepatitis (fatty liver disease) (NASH).
Start Date17 Mar 2021 |
Sponsor / Collaborator |
NCT04225936
An Open-label, Two-Part, Single-dose Study to Evaluate the Pharmacokinetics, Safety, and Tolerability of BMS-986263 in Participants With Varying Degrees of Hepatic Impairment
The primary purpose of this study is to evaluate the effect of liver impairment on the safety and pharmacokinetics (PK) of BMS-986263
Start Date16 Jan 2020 |
Sponsor / Collaborator |
NCT03538301
A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety, Tolerability, Biological Activity, and PK of ND-L02-s0201 in Subjects With Idiopathic Pulmonary Fibrosis (IPF)
A phase 2, randomized, double-blind, placebo-controlled, multicenter study to evaluate the safety, tolerability, biological activity, and pharmacokinetics (PK) of ND-L02-s0201 for Injection in subjects with IPF.
Start Date18 Jun 2018 |
Sponsor / Collaborator |
100 Clinical Results associated with BMS-986263
Login to view more data
100 Translational Medicine associated with BMS-986263
Login to view more data
100 Patents (Medical) associated with BMS-986263
Login to view more data
3
Literatures (Medical) associated with BMS-98626301 Oct 2023·Clinical and translational science
Pharmacokinetics, safety, and tolerability of BMS‐986263, a lipid nanoparticle containing HSP47 siRNA, in participants with hepatic impairment
Article
Author: Lawitz, Eric J. ; Charles, Edgar D. ; Tirucherai, Giridhar S. ; Wetherington, Jeffrey ; Cizza, Giovanni ; Qosa, Hisham ; Colletti, Nicholas ; de Oliveira, Claudia H. M. C.
Abstract:
BMS‐986263 is a retinoid‐conjugated lipid nanoparticle delivering small interfering RNA designed to inhibit synthesis of HSP47 protein, a collagen‐specific chaperone protein involved in fibrosis development. This is a phase I, open‐label, two‐part study evaluating pharmacokinetics and safety of BMS‐986263 in participants with hepatic impairment (HI). Part 1 (n = 24) of this study enrolled two cohorts with mild and moderate HI and a separate cohort of age‐ and body mass index (BMI)‐matched participants with normal hepatic function. Part 2 enrolled eight participants with severe HI and eight age‐ and BMI‐matched participants with normal hepatic function. All participants received a single intravenous 90 mg BMS‐986263 infusion. Compared with normal‐matched participants, geometric mean area under the plasma concentration‐time curve time zero to the time of the last quantifiable concentration (AUC(0‐T)) and AUC from zero to infinity (AUC(INF)) of HSP47 siRNA were similar in participants with mild HI and 34% and 163% greater in those with moderate and severe HI, respectively, whereas the maximum plasma concentration was ~25% lower in mild and moderate HI groups but 58% higher in the severe HI group than in the normal group. Adverse events were reported by two of eight, four of eight, and three of eight participants with mild, moderate, or severe HI, respectively; none were reported in the normal‐matched group. Overall, single‐dose BMS‐986263 was generally safe and well‐tolerated and dose adjustment is not considered necessary for participants with mild or moderate HI. Although available data do not indicate that dose adjustment should be performed in patients with severe HI; the optimal posology of BMS‐986263 in patients with severe HI may be determined later in its clinical development when additional data to establish exposure‐safety/efficacy relationship becomes available.
01 Apr 2021·ERJ open research
Anti-HSP47 siRNA lipid nanoparticle ND-L02-s0201 reverses interstitial pulmonary fibrosis in preclinical rat models
Article
Author: Quimbo, Alistair ; Zabludoff, Sonya ; Ying, Wenbin ; Clamme, Jean-Pierre ; Liu, Jian ; Yao, Jiping ; Liu, Yun ; Zhang, Jun ; Xia, Fengcheng
ND-L02-s0201 is a lipid nanoparticle encapsulating an siRNA which inhibits expression of heat shock protein 47 (HSP47), a collagen-specific chaperone. Accumulated evidence demonstrates a close association between increased level of HSP47 and excessive accumulation of collagen in fibrotic diseases. Our objective was to test ND-L02-s0201 efficacy in preclinical lung fibrosis models and characterise the downstream histological and functional consequences of inhibiting the expression of HSP47.Comprehensive optimisation and characterisation of bleomycin (BLM) and silica-induced rat lung fibrosis models were conducted, which ensured progressive pathological changes were sustained throughout the study during evaluation of the anti-fibrotic potential of ND-L02-s0201.In the BLM model, we demonstrated dose-dependent and statistically significant reduction in the relative lung weight, collagen deposition and histology, and fibrosis scores following ND-L02-s0201 treatment. Lung tissue mRNA profiling demonstrated that 11 out of 84 fibrosis-relevant genes were upregulated following BLM induction and were downregulated by approximately 4.5-fold following ND-L02-s0201 treatment. Epithelial–mesenchymal transition was characterised in the BLM model following ND-L02-s0201 treatment. Cell enrichment demonstrated that myofibroblasts contained the highest HSP47 mRNA expression. BLM led to more than a five-fold increase in myofibroblasts and ND-L02-s0201 treatment reduced the myofibroblasts to sham levels. Statistically significant improvement in lung function was noted in the BLM model which was determined by running endurance capacity using a 7-minute treadmill test. Comparable anti-fibrotic efficacy was also observed in the silica model.Results from two robust chronic rodent models of pulmonary fibrosis demonstrated significant anti-fibrotic effects and improved lung function which support the evaluation of ND-L02-s0201 in subjects with idiopathic pulmonary fibrosis.
01 Sep 2019·AAPS Journal
A Fit-for-Purpose Method for the Detection of Human Antibodies to Surface-Exposed Components of BMS-986263, a Lipid Nanoparticle-Based Drug Product Containing a siRNA Drug Substance
Article
Author: Kavita, Uma ; Ji, Qin C ; Pillutla, Renuka C ; Miller, Wendy
ND-L02-s0201/BMS-986263 is a lipid nanoparticle (LNP) drug product containing a heat shock protein 47 (HSP47)-specific small interfering ribonucleic acid (siRNA) and being developed for the treatment of liver and idiopathic pulmonary fibrosis. To address immunogenicity-related issues, we developed a robust, fit-for-purpose (FFP) three-tier electrochemiluminescent (ECL) anti-drug antibody (ADA) assay for the detection of antibodies (Abs) generated to surface-exposed components of BMS-986263. The drug was coated directly on plates, and several Abs specific for polyethylene glycol (PEG) and other surface components were tested for use as positive quality controls (QCs). Following selection of a rabbit monoclonal anti-PEG Ab, the assay was optimized, and various method development challenges specific to the modality and pseudo surrogate rabbit control were addressed. Screening, confirmatory, and titer cut points were validated following a statistical evaluation of 41 individual K2EDTA human plasma samples at a minimum required dilution (MRD) of 100. Assay precision, sensitivity, selectivity, drug tolerance, and hook effect were determined for the rabbit Ab prepared in human K2EDTA plasma matrix. The assay was used to interrogate anti-drug Ab (ADA) responses in normal human subjects who were administered 90 mg of the drug intravenously (IV) once every week for 3 weeks in phase I clinical trials. All pre- and post-dose samples were found to be negative for ADA. Based on these results, we concluded that BMS-986263 is not immunogenic. To the best of our knowledge, this work represents the first ADA method developed and reported for an LNP-based drug product.
5
News (Medical) associated with BMS-98626303 Jan 2024
Boehringer will use Ribo’s RIBO-GalSTAR platform to identify and develop therapies for NASH. Credit: Michael Vi via Shutterstock.
Boehringer Ingelheim is intensifying efforts to combat non-alcoholic steatohepatitis (NASH) through a new multi-target collaboration with Suzhou Ribo Life Science and Ribocure Pharmaceuticals (Ribo), where it plans to develop small interfering RNA (siRNA)-based treatments.
Free Report
How is the Biopharmaceutical industry evolving?
2021 was a year of continued innovation and change in the Biopharmaceutical industry. As the COVID-19 pandemic continues to take its toll on businesses worldwide, it’s time to look for new ways to create value, prepare for the future, and remain competitive in the ever-changing landscape.
GlobalData’s expansive report examines the business environment and trends that shape the Biopharmaceutical industry. We highlight the most impactful emerging technologies, as well as the industry, regulatory, and macroeconomic factors that influence growth prospects.
Access the report to:
Benchmark the impact of major themes on the Biopharmaceutical industry.
Gain a deeper "on the ground" perspective through exclusive opinions and analysis from industry respondents.
Evaluate the effects of COVID-19 on the sector.
Download the full report to understand what to expect and how to align your strategies for success.
Submit
Country Code *
UK (+44)
USA (+1)
Algeria (+213)
Andorra (+376)
Angola (+244)
Anguilla (+1264)
Antigua & Barbuda (+1268)
Argentina (+54)
Armenia (+374)
Aruba (+297)
Australia (+61)
Austria (+43)
Azerbaijan (+994)
Bahamas (+1242)
Bahrain (+973)
Bangladesh (+880)
Barbados (+1246)
Belarus (+375)
Belgium (+32)
Belize (+501)
Benin (+229)
Bermuda (+1441)
Bhutan (+975)
Bolivia (+591)
Bosnia Herzegovina (+387)
Botswana (+267)
Brazil (+55)
Brunei (+673)
Bulgaria (+359)
Burkina Faso (+226)
Burundi (+257)
Cambodia (+855)
Cameroon (+237)
Canada (+1)
Cape Verde Islands (+238)
Cayman Islands (+1345)
Central African Republic (+236)
Chile (+56)
China (+86)
Colombia (+57)
Comoros (+269)
Congo (+242)
Cook Islands (+682)
Costa Rica (+506)
Croatia (+385)
Cuba (+53)
Cyprus North (+90392)
Cyprus South (+357)
Czech Republic (+42)
Denmark (+45)
Djibouti (+253)
Dominica (+1809)
Dominican Republic (+1809)
Ecuador (+593)
Egypt (+20)
El Salvador (+503)
Equatorial Guinea (+240)
Eritrea (+291)
Estonia (+372)
Ethiopia (+251)
Falkland Islands (+500)
Faroe Islands (+298)
Fiji (+679)
Finland (+358)
France (+33)
French Guiana (+594)
French Polynesia (+689)
Gabon (+241)
Gambia (+220)
Georgia (+7880)
Germany (+49)
Ghana (+233)
Gibraltar (+350)
Greece (+30)
Greenland (+299)
Grenada (+1473)
Guadeloupe (+590)
Guam (+671)
Guatemala (+502)
Guinea (+224)
Guinea - Bissau (+245)
Guyana (+592)
Haiti (+509)
Honduras (+504)
Hong Kong (+852)
Hungary (+36)
Iceland (+354)
India (+91)
Indonesia (+62)
Iran (+98)
Iraq (+964)
Ireland (+353)
Israel (+972)
Italy (+39)
Jamaica (+1876)
Japan (+81)
Jordan (+962)
Kazakhstan (+7)
Kenya (+254)
Kiribati (+686)
Korea North (+850)
Korea South (+82)
Kuwait (+965)
Kyrgyzstan (+996)
Laos (+856)
Latvia (+371)
Lebanon (+961)
Lesotho (+266)
Liberia (+231)
Libya (+218)
Liechtenstein (+417)
Lithuania (+370)
Luxembourg (+352)
Macao (+853)
Macedonia (+389)
Madagascar (+261)
Malawi (+265)
Malaysia (+60)
Maldives (+960)
Mali (+223)
Malta (+356)
Marshall Islands (+692)
Martinique (+596)
Mauritania (+222)
Mayotte (+269)
Mexico (+52)
Micronesia (+691)
Moldova (+373)
Monaco (+377)
Mongolia (+976)
Montserrat (+1664)
Morocco (+212)
Mozambique (+258)
Myanmar (+95)
Namibia (+264)
Nauru (+674)
Nepal (+977)
Netherlands (+31)
New Caledonia (+687)
New Zealand (+64)
Nicaragua (+505)
Niger (+227)
Nigeria (+234)
Niue (+683)
Norfolk Islands (+672)
Northern Marianas (+670)
Norway (+47)
Oman (+968)
Palau (+680)
Panama (+507)
Papua New Guinea (+675)
Paraguay (+595)
Peru (+51)
Philippines (+63)
Poland (+48)
Portugal (+351)
Puerto Rico (+1787)
Qatar (+974)
Reunion (+262)
Romania (+40)
Russia (+7)
Rwanda (+250)
San Marino (+378)
Sao Tome & Principe (+239)
Saudi Arabia (+966)
Senegal (+221)
Serbia (+381)
Seychelles (+248)
Sierra Leone (+232)
Singapore (+65)
Slovak Republic (+421)
Slovenia (+386)
Solomon Islands (+677)
Somalia (+252)
South Africa (+27)
Spain (+34)
Sri Lanka (+94)
St. Helena (+290)
St. Kitts (+1869)
St. Lucia (+1758)
Sudan (+249)
Suriname (+597)
Swaziland (+268)
Sweden (+46)
Switzerland (+41)
Syria (+963)
Taiwan (+886)
Tajikstan (+7)
Thailand (+66)
Togo (+228)
Tonga (+676)
Trinidad & Tobago (+1868)
Tunisia (+216)
Turkey (+90)
Turkmenistan (+7)
Turkmenistan (+993)
Turks & Caicos Islands (+1649)
Tuvalu (+688)
Uganda (+256)
Ukraine (+380)
United Arab Emirates (+971)
Uruguay (+598)
Uzbekistan (+7)
Vanuatu (+678)
Vatican City (+379)
Venezuela (+58)
Vietnam (+84)
Virgin Islands - British (+1284)
Virgin Islands - US (+1340)
Wallis & Futuna (+681)
Yemen (North)(+969)
Yemen (South)(+967)
Zambia (+260)
Zimbabwe (+263)
Country *
UK
USA
Afghanistan
Åland Islands
Albania
Algeria
American Samoa
Andorra
Angola
Anguilla
Antarctica
Antigua and Barbuda
Argentina
Armenia
Aruba
Australia
Austria
Azerbaijan
Bahamas
Bahrain
Bangladesh
Barbados
Belarus
Belgium
Belize
Benin
Bermuda
Bhutan
Bolivia
Bonaire, Sint Eustatius and Saba
Bosnia and Herzegovina
Botswana
Bouvet Island
Brazil
British Indian Ocean Territory
Brunei Darussalam
Bulgaria
Burkina Faso
Burundi
Cambodia
Cameroon
Canada
Cape Verde
Cayman Islands
Central African Republic
Chad
Chile
China
Christmas Island
Cocos Islands
Colombia
Comoros
Congo
Democratic Republic of
the Congo
Cook Islands
Costa Rica
Côte d"Ivoire
Croatia
Cuba
Curaçao
Cyprus
Czech Republic
Denmark
Djibouti
Dominica
Dominican Republic
Ecuador
Egypt
El Salvador
Equatorial Guinea
Eritrea
Estonia
Ethiopia
Falkland Islands
Faroe Islands
Fiji
Finland
France
French Guiana
French Polynesia
French Southern Territories
Gabon
Gambia
Georgia
Germany
Ghana
Gibraltar
Greece
Greenland
Grenada
Guadeloupe
Guam
Guatemala
Guernsey
Guinea
Guinea-Bissau
Guyana
Haiti
Heard Island and McDonald Islands
Holy See
Honduras
Hong Kong
Hungary
Iceland
India
Indonesia
Iran
Iraq
Ireland
Isle of Man
Israel
Italy
Jamaica
Japan
Jersey
Jordan
Kazakhstan
Kenya
Kiribati
North Korea
South Korea
Kuwait
Kyrgyzstan
Lao
Latvia
Lebanon
Lesotho
Liberia
Libyan Arab Jamahiriya
Liechtenstein
Lithuania
Luxembourg
Macao
Macedonia, The Former
Yugoslav Republic of
Madagascar
Malawi
Malaysia
Maldives
Mali
Malta
Marshall Islands
Martinique
Mauritania
Mauritius
Mayotte
Mexico
Micronesia
Moldova
Monaco
Mongolia
Montenegro
Montserrat
Morocco
Mozambique
Myanmar
Namibia
Nauru
Nepal
Netherlands
New Caledonia
New Zealand
Nicaragua
Niger
Nigeria
Niue
Norfolk Island
Northern Mariana Islands
Norway
Oman
Pakistan
Palau
Palestinian Territory
Panama
Papua New Guinea
Paraguay
Peru
Philippines
Pitcairn
Poland
Portugal
Puerto Rico
Qatar
Réunion
Romania
Russian Federation
Rwanda
Saint Helena, Ascension and Tristan da Cunha
Saint Kitts and Nevis
Saint Lucia
Saint Pierre and Miquelon
Saint Vincent and The Grenadines
Samoa
San Marino
Sao Tome and Principe
Saudi Arabia
Senegal
Serbia
Seychelles
Sierra Leone
Singapore
Slovakia
Slovenia
Solomon Islands
Somalia
South Africa
South Georgia and The South
Sandwich Islands
Spain
Sri Lanka
Sudan
Suriname
Svalbard and Jan Mayen
Swaziland
Sweden
Switzerland
Syrian Arab Republic
Taiwan
Tajikistan
Tanzania
Thailand
Timor-Leste
Togo
Tokelau
Tonga
Trinidad and Tobago
Tunisia
Turkey
Turkmenistan
Turks and Caicos Islands
Tuvalu
Uganda
Ukraine
United Arab Emirates
US Minor Outlying Islands
Uruguay
Uzbekistan
Vanuatu
Venezuela
Vietnam
British Virgin Islands
US Virgin Islands
Wallis and Futuna
Western Sahara
Yemen
Zambia
Zimbabwe
Kosovo
By downloading this case study, you acknowledge that GlobalData may share your information with
GlobalData
and that your personal data will be used as described in their
Privacy Policy
Submit
Visit our
Privacy Policy
for more information about our services, how GlobalData may use, process and share your personal data, including information on your rights in respect of your personal data and how you can unsubscribe from future marketing communications. Our services are intended for corporate subscribers and you warrant that the email address submitted is your corporate email address.
Thank you.
Please check your email to download the Report.
Go deeper with GlobalData
Reports
NPVM: Bristol-Myers Squibb Co's BMS-986263
Reports
Non-Alcoholic Steatohepatitis - Epidemiology Forecast to 2029
Premium Insights
The gold standard of business intelligence.
Find out more
Related Company Profiles
Boehringer Ingelheim International GmbH
Dicerna Pharmaceuticals Inc
Novo Nordisk AS
Zealand Pharma AS
View all
The agreement’s value is set to potentially exceed $2bn and will allow Boehringer to capitalise on Ribo’s RIBO-GalSTAR platform for targeted siRNA therapeutics in liver cells.
This collaboration comes six years after
Boehringer joined forces
with
Dicerna Pharmaceuticals
to discover and develop new therapeutics using Dicerna’s GalXC technology platform, for the treatment of liver diseases including NASH. Under the terms of the deal, Dicerna received over $200m from Boehringer, including an upfront payment, and development and commercial milestone payments.
In May 2021, Dicerna announced that an siRNA candidate called DCR-LIV2 conjugated with a hepatocyte-targeting ligand was accepted by Boehringer for development, but the latter company has not announced any updates on its progress since then. Later, in November 2021,
Novo Nordisk
acquired
Dicerna in a $3.3bn transaction that gave Novo access to Dicerna’s RNAi platform.
Earlier in 2017, Boehringer
signed an agreement
with MiNA Therapeutics, developing compounds to target NASH. At the time, MiNA received an upfront payment of $307m from Boehringer. As per GlobalData, MiNA has a small activating RNA (saRNA) therapeutic in the discovery stage for NASH.
GlobalData is the parent company of
Pharmaceutical Technology
.
NASH is a form of non-alcoholic fatty liver disease characterised by inflammation and liver cell damage, often associated with the accumulation of fat in the liver. Unlike simple fatty liver, NASH can progress into more severe conditions including cirrhosis and liver cancer. Historically, it has been challenging to develop any effective treatments for NASH with the field being littered with several high-profile failures. Still, there is a limited, but
steady pipeline of candidates
that continue to be explored.
In the statement accompanying the partnership, senior vice president and global head of cardiometabolic diseases research at Boehringer Søren Tullin said: “This new partnership is part of our commitment to collaborate with peers worldwide to address the interconnected nature of CRM diseases. Our goal is to develop the next wave of innovative medicines that will lead to a holistic health gain for patients.”
In addition to NASH, Boehringer is targeting obesity, with the German company
releasing
positive Phase II data for survodutide, a subcutaneous long-acting dual glucagon and GLP-1 agonist which is being developed in partnership with
Zealand Pharma
, in June of last year. The drug is meant to treat individuals with a body mass index (BMI) above 27kg/m², and NASH.
According to a patient-based forecast on GlobalData’s Pharma Intelligence Center, survodutide is predicted to generate $707m in 2030.
Free Report
How is the Biopharmaceutical industry evolving?
2021 was a year of continued innovation and change in the Biopharmaceutical industry. As the COVID-19 pandemic continues to take its toll on businesses worldwide, it’s time to look for new ways to create value, prepare for the future, and remain competitive in the ever-changing landscape.
GlobalData’s expansive report examines the business environment and trends that shape the Biopharmaceutical industry. We highlight the most impactful emerging technologies, as well as the industry, regulatory, and macroeconomic factors that influence growth prospects.
Access the report to:
Benchmark the impact of major themes on the Biopharmaceutical industry.
Gain a deeper "on the ground" perspective through exclusive opinions and analysis from industry respondents.
Evaluate the effects of COVID-19 on the sector.
Download the full report to understand what to expect and how to align your strategies for success.
By GlobalData
Submit
Country Code *
UK (+44)
USA (+1)
Algeria (+213)
Andorra (+376)
Angola (+244)
Anguilla (+1264)
Antigua & Barbuda (+1268)
Argentina (+54)
Armenia (+374)
Aruba (+297)
Australia (+61)
Austria (+43)
Azerbaijan (+994)
Bahamas (+1242)
Bahrain (+973)
Bangladesh (+880)
Barbados (+1246)
Belarus (+375)
Belgium (+32)
Belize (+501)
Benin (+229)
Bermuda (+1441)
Bhutan (+975)
Bolivia (+591)
Bosnia Herzegovina (+387)
Botswana (+267)
Brazil (+55)
Brunei (+673)
Bulgaria (+359)
Burkina Faso (+226)
Burundi (+257)
Cambodia (+855)
Cameroon (+237)
Canada (+1)
Cape Verde Islands (+238)
Cayman Islands (+1345)
Central African Republic (+236)
Chile (+56)
China (+86)
Colombia (+57)
Comoros (+269)
Congo (+242)
Cook Islands (+682)
Costa Rica (+506)
Croatia (+385)
Cuba (+53)
Cyprus North (+90392)
Cyprus South (+357)
Czech Republic (+42)
Denmark (+45)
Djibouti (+253)
Dominica (+1809)
Dominican Republic (+1809)
Ecuador (+593)
Egypt (+20)
El Salvador (+503)
Equatorial Guinea (+240)
Eritrea (+291)
Estonia (+372)
Ethiopia (+251)
Falkland Islands (+500)
Faroe Islands (+298)
Fiji (+679)
Finland (+358)
France (+33)
French Guiana (+594)
French Polynesia (+689)
Gabon (+241)
Gambia (+220)
Georgia (+7880)
Germany (+49)
Ghana (+233)
Gibraltar (+350)
Greece (+30)
Greenland (+299)
Grenada (+1473)
Guadeloupe (+590)
Guam (+671)
Guatemala (+502)
Guinea (+224)
Guinea - Bissau (+245)
Guyana (+592)
Haiti (+509)
Honduras (+504)
Hong Kong (+852)
Hungary (+36)
Iceland (+354)
India (+91)
Indonesia (+62)
Iran (+98)
Iraq (+964)
Ireland (+353)
Israel (+972)
Italy (+39)
Jamaica (+1876)
Japan (+81)
Jordan (+962)
Kazakhstan (+7)
Kenya (+254)
Kiribati (+686)
Korea North (+850)
Korea South (+82)
Kuwait (+965)
Kyrgyzstan (+996)
Laos (+856)
Latvia (+371)
Lebanon (+961)
Lesotho (+266)
Liberia (+231)
Libya (+218)
Liechtenstein (+417)
Lithuania (+370)
Luxembourg (+352)
Macao (+853)
Macedonia (+389)
Madagascar (+261)
Malawi (+265)
Malaysia (+60)
Maldives (+960)
Mali (+223)
Malta (+356)
Marshall Islands (+692)
Martinique (+596)
Mauritania (+222)
Mayotte (+269)
Mexico (+52)
Micronesia (+691)
Moldova (+373)
Monaco (+377)
Mongolia (+976)
Montserrat (+1664)
Morocco (+212)
Mozambique (+258)
Myanmar (+95)
Namibia (+264)
Nauru (+674)
Nepal (+977)
Netherlands (+31)
New Caledonia (+687)
New Zealand (+64)
Nicaragua (+505)
Niger (+227)
Nigeria (+234)
Niue (+683)
Norfolk Islands (+672)
Northern Marianas (+670)
Norway (+47)
Oman (+968)
Palau (+680)
Panama (+507)
Papua New Guinea (+675)
Paraguay (+595)
Peru (+51)
Philippines (+63)
Poland (+48)
Portugal (+351)
Puerto Rico (+1787)
Qatar (+974)
Reunion (+262)
Romania (+40)
Russia (+7)
Rwanda (+250)
San Marino (+378)
Sao Tome & Principe (+239)
Saudi Arabia (+966)
Senegal (+221)
Serbia (+381)
Seychelles (+248)
Sierra Leone (+232)
Singapore (+65)
Slovak Republic (+421)
Slovenia (+386)
Solomon Islands (+677)
Somalia (+252)
South Africa (+27)
Spain (+34)
Sri Lanka (+94)
St. Helena (+290)
St. Kitts (+1869)
St. Lucia (+1758)
Sudan (+249)
Suriname (+597)
Swaziland (+268)
Sweden (+46)
Switzerland (+41)
Syria (+963)
Taiwan (+886)
Tajikstan (+7)
Thailand (+66)
Togo (+228)
Tonga (+676)
Trinidad & Tobago (+1868)
Tunisia (+216)
Turkey (+90)
Turkmenistan (+7)
Turkmenistan (+993)
Turks & Caicos Islands (+1649)
Tuvalu (+688)
Uganda (+256)
Ukraine (+380)
United Arab Emirates (+971)
Uruguay (+598)
Uzbekistan (+7)
Vanuatu (+678)
Vatican City (+379)
Venezuela (+58)
Vietnam (+84)
Virgin Islands - British (+1284)
Virgin Islands - US (+1340)
Wallis & Futuna (+681)
Yemen (North)(+969)
Yemen (South)(+967)
Zambia (+260)
Zimbabwe (+263)
Country *
UK
USA
Afghanistan
Åland Islands
Albania
Algeria
American Samoa
Andorra
Angola
Anguilla
Antarctica
Antigua and Barbuda
Argentina
Armenia
Aruba
Australia
Austria
Azerbaijan
Bahamas
Bahrain
Bangladesh
Barbados
Belarus
Belgium
Belize
Benin
Bermuda
Bhutan
Bolivia
Bonaire, Sint Eustatius and Saba
Bosnia and Herzegovina
Botswana
Bouvet Island
Brazil
British Indian Ocean Territory
Brunei Darussalam
Bulgaria
Burkina Faso
Burundi
Cambodia
Cameroon
Canada
Cape Verde
Cayman Islands
Central African Republic
Chad
Chile
China
Christmas Island
Cocos Islands
Colombia
Comoros
Congo
Democratic Republic of
the Congo
Cook Islands
Costa Rica
Côte d"Ivoire
Croatia
Cuba
Curaçao
Cyprus
Czech Republic
Denmark
Djibouti
Dominica
Dominican Republic
Ecuador
Egypt
El Salvador
Equatorial Guinea
Eritrea
Estonia
Ethiopia
Falkland Islands
Faroe Islands
Fiji
Finland
France
French Guiana
French Polynesia
French Southern Territories
Gabon
Gambia
Georgia
Germany
Ghana
Gibraltar
Greece
Greenland
Grenada
Guadeloupe
Guam
Guatemala
Guernsey
Guinea
Guinea-Bissau
Guyana
Haiti
Heard Island and McDonald Islands
Holy See
Honduras
Hong Kong
Hungary
Iceland
India
Indonesia
Iran
Iraq
Ireland
Isle of Man
Israel
Italy
Jamaica
Japan
Jersey
Jordan
Kazakhstan
Kenya
Kiribati
North Korea
South Korea
Kuwait
Kyrgyzstan
Lao
Latvia
Lebanon
Lesotho
Liberia
Libyan Arab Jamahiriya
Liechtenstein
Lithuania
Luxembourg
Macao
Macedonia, The Former
Yugoslav Republic of
Madagascar
Malawi
Malaysia
Maldives
Mali
Malta
Marshall Islands
Martinique
Mauritania
Mauritius
Mayotte
Mexico
Micronesia
Moldova
Monaco
Mongolia
Montenegro
Montserrat
Morocco
Mozambique
Myanmar
Namibia
Nauru
Nepal
Netherlands
New Caledonia
New Zealand
Nicaragua
Niger
Nigeria
Niue
Norfolk Island
Northern Mariana Islands
Norway
Oman
Pakistan
Palau
Palestinian Territory
Panama
Papua New Guinea
Paraguay
Peru
Philippines
Pitcairn
Poland
Portugal
Puerto Rico
Qatar
Réunion
Romania
Russian Federation
Rwanda
Saint Helena, Ascension and Tristan da Cunha
Saint Kitts and Nevis
Saint Lucia
Saint Pierre and Miquelon
Saint Vincent and The Grenadines
Samoa
San Marino
Sao Tome and Principe
Saudi Arabia
Senegal
Serbia
Seychelles
Sierra Leone
Singapore
Slovakia
Slovenia
Solomon Islands
Somalia
South Africa
South Georgia and The South
Sandwich Islands
Spain
Sri Lanka
Sudan
Suriname
Svalbard and Jan Mayen
Swaziland
Sweden
Switzerland
Syrian Arab Republic
Taiwan
Tajikistan
Tanzania
Thailand
Timor-Leste
Togo
Tokelau
Tonga
Trinidad and Tobago
Tunisia
Turkey
Turkmenistan
Turks and Caicos Islands
Tuvalu
Uganda
Ukraine
United Arab Emirates
US Minor Outlying Islands
Uruguay
Uzbekistan
Vanuatu
Venezuela
Vietnam
British Virgin Islands
US Virgin Islands
Wallis and Futuna
Western Sahara
Yemen
Zambia
Zimbabwe
Kosovo
By downloading this case study, you acknowledge that GlobalData may share your information with
GlobalData
and that your personal data will be used as described in their
Privacy Policy
Submit
Visit our
Privacy Policy
for more information about our services, how GlobalData may use, process and share your personal data, including information on your rights in respect of your personal data and how you can unsubscribe from future marketing communications. Our services are intended for corporate subscribers and you warrant that the email address submitted is your corporate email address.
Thank you.
Please check your email to download the Report.
AcquisitionPhase 2
14 Sep 2023
Bristol Myers Squibb is ending development of a handful of new assets, including an anti-TIGIT med in phase 2.
As the TIGIT class gets closer to finally seeing clinical success, Bristol Myers Squibb is trimming one of its candidates.
The company disclosed development of a phase 2 anti-TIGIT is ending as part of its annual R&D day on Thursday. BMS is also halting work on a phase 2 nonalcoholic steatohepatitis (NASH) candidate licensed in 2016 from Nitto Denko Corporation and a handful of other phase 1 assets.
"We regularly evaluate our pipeline to best prioritize resources where we see the potential for transformational effects on patients’ lives," a spokesperson said in a statement.
BMS terminated a phase 2 study of the anti-TIGIT in solid tumors in late January due to “safety reasons, adverse change in the risk/benefit,” according to the clinical trial record. The record for a phase 1/2 multiple myeloma trial, sponsored by the Multiple Myeloma Research Consortium, was last updated in July to indicate it was active and recruitment had been completed.
The decision comes as the class is potentially on the precipice of a bit of momentum, led by Roche's tiragolumab. Data released last year found that the asset in combination with Tecentriq did not improve progression-free survival in first-line non-small cell lung cancer patients, throwing into question the potential of the class that had garnered investments from numerous Big Pharmas. But an inadvertent data drop last month showed that treated patients did live longer compared to patients on just Tecentriq, though not by a statistically significant amount. Nonetheless, Roche's shared jump on the news.
BMS is evidently pushing all of its TIGIT chips toward a bispecific candidate licensed from Agenus in May 2021 for $200 million upfront. Agenus also stands to earn $1.36 billion in milestone payments. The bispecific is in a phase 1 trial in patients with solid tumors as both a monotherapy and in combination with a PD-1 inhibitor.
The NASH med was a siRNA asset targeting heat shock protein 47 that cost BMS $100 million to license in 2016. A phase 2 trial testing BMS-986263 in patients with advanced hepatic fibrosis that had been cured of hepatitis C wrapped up in February 2022. Data published in April last year showed that the most significant improvement in fibrosis was in patients at the 90-mg dose level, the highest in the trial, and that all adverse events were mild to moderate.
The early-stage cuts include a cancer molecule aimed at an undisclosed target, a CD47 and CD20-targeting lymphoma med, a leukemia treatment and a RIPK1 inhibitor.
Editor's note: This story was updated to include a statement from a BMS spokesperson.

Phase 1Phase 2PROTACsClinical Trial TerminationLicense out/in
25 May 2023
The prevalence of idiopathic pulmonary fibrosis has been rising over the past few years, which prompts the growing demand for treatment options. The increasing prevalence of idiopathic pulmonary fibrosis and the growing research and development activities to develop novel therapies to treat idiopathic pulmonary fibrosis to drive the market. The companies developing the potential therapies in the last stage of development include
FibroGen, Boehringer Ingelheim, and several others.
LAS VEGAS, May 25, 2023 /PRNewswire/ -- DelveInsight's
'
Idiopathic Pulmonary Fibrosis Pipeline Insight – 2023
' report provides comprehensive global coverage of pipeline idiopathic pulmonary fibrosis therapies in various stages of clinical development, major pharmaceutical companies working to advance the pipeline space, and future growth potential of the idiopathic pulmonary fibrosis pipeline domain.
Key Takeaways from the Idiopathic Pulmonary Fibrosis Pipeline Report
DelveInsight's idiopathic pulmonary fibrosis pipeline report depicts a robust space with
80+ active players working to develop
100+ pipeline therapies for idiopathic pulmonary fibrosis treatment.
Key idiopathic pulmonary fibrosis companies such as
FibroGen, United Therapeutics, Bellerophon Therapeutics, MediciNova, Novartis, Endeavor BioMedicines, Pliant Therapeutics, Nitto Denko, Kadmon Pharmaceuticals, Calliditas Therapeutics, Avalyn Pharmaceuticals, PureTech Health, Taiho Pharmaceutical, Bristol-Myers Squibb, Galecto Biotech AB, CSL Behring, Celgene Pharmaceutical, Vicore Pharma, Boehringer Ingelheim, Guangdong Raynovent, Sunshine Lake Pharma co, Suzhou Zelgen Biopharmaceuticals, Algernon Pharmaceuticals, Horizon Therapeutics, Daewoong Pharmaceutical, Metagone Biotech, AstraZeneca, Lung Therapeutics, Bridge Biotherapeutics, AstraZeneca, Kinarus AG, Insmed, Reviva Pharmaceuticals, Annapurna Bio, Guangdong Hengrui Pharmaceutical Co., Ltd, Ark Biosciences, Ocean Biomedical, and others are evaluating new idiopathic pulmonary fibrosis drugs to improve the treatment landscape.
Promising idiopathic pulmonary fibrosis pipeline therapies in various stages of development include
Pamrevlumab, Treprostinil, Nitric oxide inhalation - INOpulse, MN-001 (tipelukast), VAY736, ENV-101, PLN-74809, ND-L02-s0201, KD025, GKT137831, AP 01, LYT-100, TAS-115, BMS-986278, GB0139, CSL312, CC-90001, C21, BI1015550, ZSP1603, HEC585, Jaktinib Dihydrochloride Monohydrate, Ifenprodil, HZN-825, DWN12088, MG-S-2525, Saracatinib, LTI-03, BBT-877, AZD5055, KIN001-IPF, Treprostinil palmitil inhalation powder (TPIP), Brilaroxazine, ANPA 0073, SHR 1906, AK 3280, OCF 203, and others.
In
May 2023, Kinarus Therapeutics announced the signing of a strategic convertible loan agreement for a
CHF 1.5 million investment by
ChaoDian (Hangzhou) Investment Management Co., Ltd., an investment company based in Hangzhou City, China ("CDIM"). Further, this agreement forms the basis for discussions on the introduction, development and commercialization of
KIN001 for the treatment of Idiopathic Pulmonary Fibrosis (IPF) in China. Great Health Companion Group Ltd (GHCG), a subsidiary of Hakim Unique Group, introduced CDIM to Kinarus.
In April 2023, AGC Biologics announced it signed a service agreement with
The Jikei University in Japan. Under the agreement, AGC Biologics will assume a technology transfer and feasibility study for a drug product focused on the treatment of Idiopathic pulmonary fibrosis at the CDMO's center of Cell and Gene Excellence in Milan.
In February 2023, Insilico Medicine announced that the US Food and Drug Administration (FDA) had granted
Orphan Drug Designation to
INS018_055 for the treatment of Idiopathic Pulmonary Fibrosis (IPF).
In February 2023, Arrowhead Pharmaceuticals Inc. announced that it had dosed the first subjects in a Phase I/IIa clinical trial of ARO-MMP7, the company's investigational RNA interference (RNAi) therapeutic designed to reduce the expression of matrix metalloproteinase 7 (MMP7) as a potential treatment for idiopathic pulmonary fibrosis (IPF).
In February 2023, South Korea's Daewoong Pharmaceutical secured an exclusive licensing agreement with
CS Pharmaceuticals for a first-in-class PRS inhibitor Bersiporocin in the Greater China region, including mainland China, Hong Kong, Taiwan, and Macau. Under this agreement, CSP will in-license
Bersiporocin for Idiopathic Pulmonary Fibrosis (IPF) and potentially other fibrotic indications for a total consideration of up to
$336 million, including up to
$76 million in upfront and development milestone payments and double-digit royalties on Net Sales.
In January 2023, Insilico Medicine announced the positive topline results of safety, tolerability, and pharmacokinetics (PK) from the Phase 1 clinical trial of
INS018_055, a potential first-in-class drug discovered by Insilico's end-to-end AI platform for idiopathic pulmonary fibrosis (IPF).
In January 2023, Pliant Therapeutics announced 12-week
interim data from the 320 mg dose group of INTEGRIS-IPF, a multinational, randomized, double-blind, placebo-controlled Phase 2a clinical trial of
bexotegrast (PLN-74809) in patients with idiopathic pulmonary fibrosis (IPF).
In December 2022, Vallon Pharmaceuticals announced that they entered into a
definitive agreement (the "Merger Agreement") pursuant to which
GRI Bio will merge with a wholly-owned subsidiary of Vallon in an all-stock transaction (the "Merger"). The combined company will focus on advancing GRI Bio's innovative pipeline of NKT cell regulators for the treatment of inflammatory, fibrotic and autoimmune diseases. Following the closing of the Merger, the combined company is expected to operate under the name
"GRI Bio, Inc."
Request a sample and discover the recent advances in idiopathic pulmonary fibrosis drug treatment @
Idiopathic Pulmonary Fibrosis Pipeline Report
The idiopathic pulmonary fibrosis pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage idiopathic pulmonary fibrosis drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the idiopathic pulmonary fibrosis clinical trial landscape.
Idiopathic Pulmonary Fibrosis Overview
Idiopathic pulmonary fibrosis (IPF) is a rare, chronic, progressive fibrosing interstitial pneumonia that primarily affects middle-aged and older adults. It affects lung tissue by thickening, stiffening, or persistent and progressive scarring that worsens irreversibly over time. Scarring affects the air sacs of IPF patients, decreasing the amount of oxygen that enters the circulation. With less oxygen in the blood, ordinary tasks such as walking can cause dyspnea. This set of lung illnesses is also known as 'Diffuse Parenchymal Lung Diseases,' and it is distinguished by a broader umbrella of 'Interstitial Lung Diseases (IDLs)'. The cause of IPF is uncertain; experts believe the condition is caused by a mix of hereditary and environmental factors. There is a significant chance that genetic variations enhance a person's risk of acquiring IPF and then being exposed to environmental variables that aggravate the condition. However, much remains unknown about this new field of study.
Find out more about drugs for idiopathic pulmonary fibrosis @
New Idiopathic Pulmonary Fibrosis Drugs
A snapshot of the Idiopathic Pulmonary Fibrosis Pipeline Drugs mentioned in the report:
Learn more about the emerging idiopathic pulmonary fibrosis pipeline therapies @
Idiopathic Pulmonary Fibrosis Clinical Trials
Idiopathic Pulmonary Fibrosis Therapeutics Assessment
The idiopathic pulmonary fibrosis pipeline report proffers an integral view of idiopathic pulmonary fibrosis emerging novel therapies segmented by stage, product type, molecule type, mechanism of action, and route of administration.
Scope of the Idiopathic Pulmonary Fibrosis Pipeline Report
Coverage: Global
Idiopathic Pulmonary Fibrosis Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
Idiopathic Pulmonary Fibrosis Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
Idiopathic Pulmonary Fibrosis Therapeutics Assessment
By Route of Administration: Oral, Parenteral, Intravitreal, Subretinal, Topical
Idiopathic Pulmonary Fibrosis Therapeutics Assessment
By Molecule Type: Monoclonal Antibody, Peptides, Polymer, Small molecule, Gene therapy
Idiopathic Pulmonary Fibrosis Therapeutics Assessment
By Mechanism of Action: Connective tissue growth factor inhibitors, 5-lipoxygenase inhibitors, Leukotriene D4 receptor antagonists, Leukotriene receptor antagonists, Phospholipase C inhibitors, Thromboxane A2 receptor antagonists, Type 3 cyclic nucleotide phosphodiesterase inhibitors, Type 4 cyclic nucleotide phosphodiesterase inhibitors, Integrin alphavbeta1 inhibitors, Integrin alphaVbeta6 inhibitors, Antibody-dependent cell cytotoxicity, Interleukin 4 receptor antagonists, SMO protein inhibitors, Galectin 3 inhibitors
Key Idiopathic Pulmonary Fibrosis Companies: FibroGen, United Therapeutics, Bellerophon Therapeutics, MediciNova, Novartis, Endeavor BioMedicines, Pliant Therapeutics, Nitto Denko, Kadmon Pharmaceuticals, Calliditas Therapeutics, Avalyn Pharmaceuticals, PureTech Health, Taiho Pharmaceutical, Bristol-Myers Squibb, Galecto Biotech AB, CSL Behring, Celgene Pharmaceutical, Vicore Pharma, Boehringer Ingelheim, Guangdong Raynovent, Sunshine Lake Pharma co, Suzhou Zelgen Biopharmaceuticals, Algernon Pharmaceuticals, Horizon Therapeutics, Daewoong Pharmaceutical, Metagone Biotech, AstraZeneca, Lung Therapeutics, Bridge Biotherapeutics, AstraZeneca, Kinarus AG, Insmed, Reviva Pharmaceuticals, Annapurna Bio, Guangdong Hengrui Pharmaceutical Co., Ltd, Ark Biosciences, Ocean Biomedical, and others.
Key Idiopathic Pulmonary Fibrosis Pipeline Therapies: Pamrevlumab, Treprostinil, Nitric oxide inhalation - INOpulse, MN-001 (tipelukast), VAY736, ENV-101, PLN-74809, ND-L02-s0201, KD025, GKT137831, AP 01, LYT-100, TAS-115, BMS-986278, GB0139, CSL312, CC-90001, C21, BI1015550, ZSP1603, HEC585, Jaktinib Dihydrochloride Monohydrate, Ifenprodil, HZN-825, DWN12088, MG-S-2525, Saracatinib, LTI-03, BBT-877, AZD5055, KIN001-IPF, Treprostinil palmitil inhalation powder (TPIP), Brilaroxazine, ANPA 0073, SHR 1906, AK 3280, OCF 203, and others.
Dive deep into rich insights for new drugs for idiopathic pulmonary fibrosis treatment; visit @
Idiopathic Pulmonary Fibrosis Medications
Table of Contents
For further information on the idiopathic pulmonary fibrosis pipeline therapeutics, reach out @
Idiopathic Pulmonary Fibrosis Drug Treatment
Related Reports
Idiopathic Pulmonary Fibrosis Market
Idiopathic Pulmonary Fibrosis Market Insights, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key idiopathic pulmonary fibrosis companies, including
FibroGen, Hoffmann-La Roche Ltd, United Therapeutics, Boehringer Ingelheim, Pliant Therapeutics, Inc., Galecto Biotech, Horizon Therapeutics, CSL Behring, Kadmon Corporation, LLC, among others.
Idiopathic Pulmonary Fibrosis Epidemiology Forecast
Idiopathic Pulmonary Fibrosis Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, and idiopathic pulmonary fibrosis epidemiology trends.
Pulmonary Fibrosis Pipeline
Pulmonary Fibrosis Pipeline Insight – 2023 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key pulmonary fibrosis companies, including
Roche, Boehringer Ingelheim, Galecto Biotech, Cipla, Gilead Sciences, Fibrogen, Bristol-Myers Squibb, among others.
Pulmonary Fibrosis Market
Pulmonary Fibrosis Market Insights, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key pulmonary fibrosis companies, including
Roche, Boehringer Ingelheim, Galecto Biotech, Cipla, Gilead Sciences, Fibrogen, Bristol-Myers Squibb, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve
.
Contact Us
Shruti Thakur
[email protected]
+1(919)321-6187
Logo:
SOURCE DelveInsight Business Research, LLP
Phase 1License out/inOrphan DrugPhase 2
100 Deals associated with BMS-986263
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Metabolic Dysfunction Associated Steatohepatitis | Phase 2 | United States | 17 Mar 2021 | |
| Metabolic Dysfunction Associated Steatohepatitis | Phase 2 | Japan | 17 Mar 2021 | |
| Metabolic Dysfunction Associated Steatohepatitis | Phase 2 | Argentina | 17 Mar 2021 | |
| Metabolic Dysfunction Associated Steatohepatitis | Phase 2 | Belgium | 17 Mar 2021 | |
| Metabolic Dysfunction Associated Steatohepatitis | Phase 2 | Brazil | 17 Mar 2021 | |
| Metabolic Dysfunction Associated Steatohepatitis | Phase 2 | Canada | 17 Mar 2021 | |
| Metabolic Dysfunction Associated Steatohepatitis | Phase 2 | France | 17 Mar 2021 | |
| Metabolic Dysfunction Associated Steatohepatitis | Phase 2 | Germany | 17 Mar 2021 | |
| Metabolic Dysfunction Associated Steatohepatitis | Phase 2 | Israel | 17 Mar 2021 | |
| Metabolic Dysfunction Associated Steatohepatitis | Phase 2 | Italy | 17 Mar 2021 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 2 | 124 | Placebo | bwywyyrmmf = jbizczhsbu fqqhbaiwxp (vredijjzyf, frysgouamo - ywgelivbnv) View more | - | 04 Sep 2024 | ||
Phase 2 | 123 | Other: Placebo | lurkrpeabu = fkeqnrszzy nlkhegoknb (yvuurfgeeq, uipovgsoyo - anprnpoyew) View more | - | 11 Dec 2023 | ||
Phase 1 | 8 | clxjskyoga(giirhdkufo) = 1 in BMS-986263, 0 in normal-matched arfyajnazn (ldbfddwyaj ) View more | Positive | 25 Jun 2022 | |||
Phase 2 | 61 | (BMS-986263 45 mg QW (Once Weekly)) | hmatxynxfl = ghfafervrq xyvezzlhne (fvsohltwgs, anibfxmovy - blgdvzehal) View more | - | 04 Feb 2022 | ||
(BMS-986263 90 mg QW (Once Weekly)) | hmatxynxfl = tyiueedywd xyvezzlhne (fvsohltwgs, ythhtbudqo - pybchuhwwj) View more | ||||||
NCT03420768 (Pubmed) Manual | Phase 2 | 61 | kcdasyqxec(sorichskyh) = qhkangeqag keyovxmgsi (qiufdrmqcy ) View more | Positive | 04 Oct 2021 | ||
Placebo | kcdasyqxec(sorichskyh) = owgivslefc keyovxmgsi (qiufdrmqcy ) |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free

