/ CompletedNot Applicable Randomized Open-Label Clinical Study Evaluating the Impact of EnteraGam, a Nutritional Intervention Containing Bovine Plasma-Derived Immunoglobulin CoNcentrate, on Clinical Outcomes In People With COVID-19
Background:
Coronavirus Disease 2019 (COVID-19), caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), emerged as a potentially life-threatening disease in Wuhan, China, at the end of 2019. Since then, it has spread to almost 200 countries and infection rates are rapidly accelerating.
Overactivation of T cells resulting in immune dysfunction, dysfunction of the renin angiotensin system, and antibody-dependent enhancement are thought to contribute to the cytokine storm that results in acute respiratory distress syndrome (ARDS), culminating in death. In addition to causing respiratory symptoms, SARS-CoV-2 can cause diarrhea and has been isolated from the stool. SARS-CoV-2 binds to Angiotensin-converting enzyme 2 (ACE2) on lung alveolar type 2 cells, but ACE2 is also expressed in the absorptive enterocytes from the ileum and colon. The diarrhea may be caused by increased intestinal permeability due to binding of these receptors by the SARS-CoV-2.
Thus, an intervention to attenuate this cytokine storm may improve clinical outcomes in people with COVID-19. One such intervention is oral administration of serum bovine immunoglobulins, which decreases interleukin-6 (IL-6) levels safely with minimal side effects. Animal and human clinical studies have shown dietary supplementation with oral immunoglobulins improves mucosal immunity, specifically respiratory/pulmonary and GI mucosa, and decreases systemic inflammation, reducing the symptoms and severity of pulmonary inflammation and viral infections.
Hypothesis:
Dietary supplementation with EnteraGam® will decrease IL-6 levels and prevent disease progression in SARS-CoV-2 infected individuals.
Objectives:
To evaluate the effectiveness of the oral nutritional therapy EnteraGam® (serum-derived bovine immunoglobulin/protein isolate) to prevent disease progression of COVID-19 and to decrease IL-6 levels as compared to standard of care in subjects with COVID-19.
Methods:
Randomized open-label clinical study evaluating the effectiveness of EnteraGam® 10.0 g BID (every 12 hours) added to standard of care, as compared to standard of care alone, in subjects with COVID-19.
/ CompletedNot ApplicableIIT Evaluating the Nutritional Impact of Serum-Derived Bovine Immunoglobulin Protein Isolate in Subjects With Ileal Pouch-Anal Anastomosis
This is an open label trial to test the hypothesize that serum bovine immunoglobulin protein isolate (SBI) will improve the nutritional status and quality of life (QOL) of patients with an ileal pouch anal anastomosis (IPAA) and symptoms of pouchitis. Subjects with symptomatic IPAA will receive two packets of EnteraGam twice daily (total daily dose of 20 g SBI) for up to 24 weeks. The primary objective of this study is to determine whether SBI therapy leads to improved nutritional status and QOL. A secondary objective is to evaluate SBI in the management of their disease, including impact on clinical symptoms.
A Randomized, Placebo-Controlled, Pilot Study of Colesevelam and Serum-Derived Bovine Immunoglobulin/Protein Isolate to Manage Diarrhea in Patients With Multiple Myeloma Receiving Conditioning Chemotherapy for Autologous Stem Cell Transplantation (SCT)
For patients who receive a hematopoietic cell transplant (HCT), there is a risk of developing a diarrhea secondary to the chemotherapy which we give. Diarrhea is usually harmless in healthy adults; however, in transplant patients, diarrhea can result in dehydration, negative impact on quality of life, and prolonged hospitalization. The purpose of this study was to see if Colesevelam (Welchol) and Serum-derived bovine immunoglobulin-protein (SBI) result in a change in the frequency or consistency of your bowel movements.
100 Clinical Results associated with Entera Health
0 Patents (Medical) associated with Entera Health
100 Deals associated with Entera Health
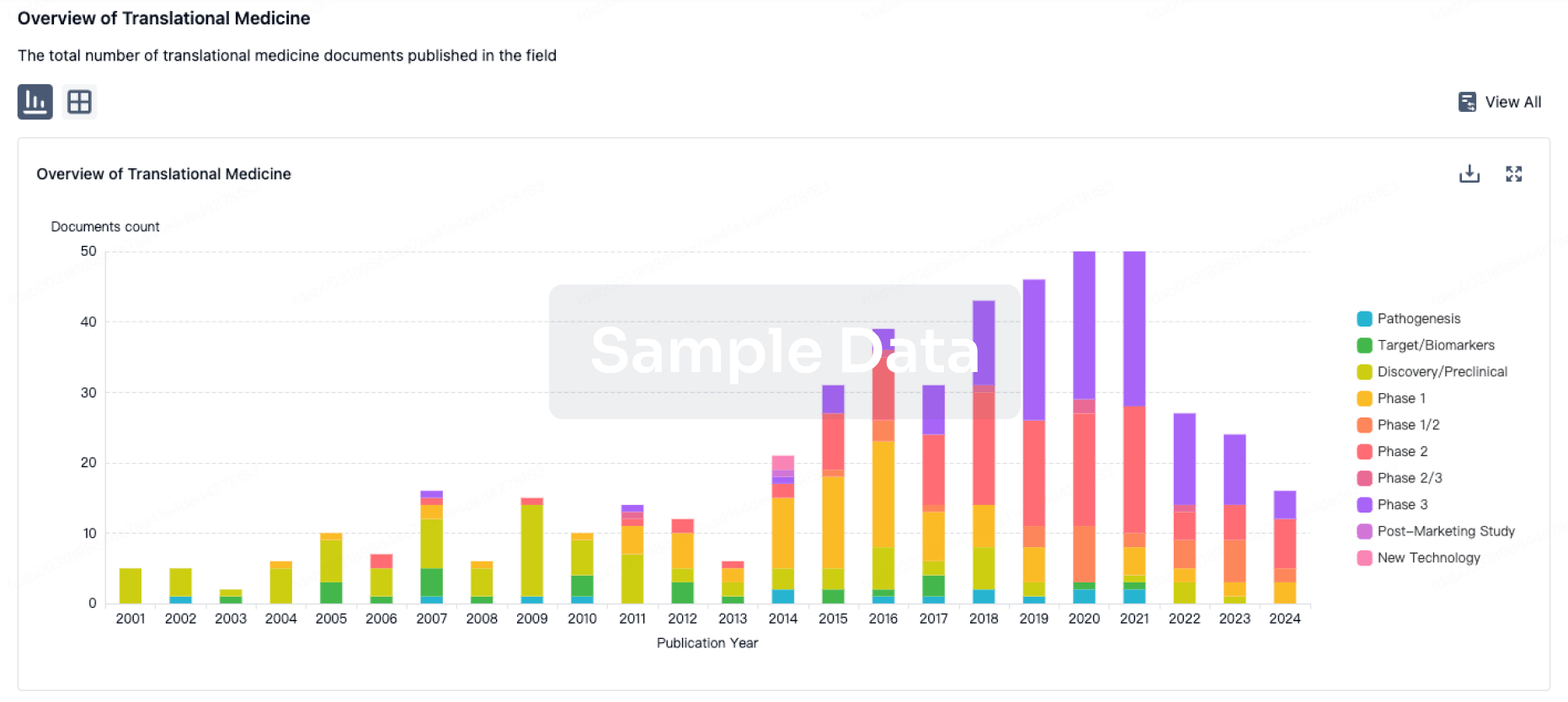
100 Translational Medicine associated with Entera Health