Request Demo
Last update 22 Dec 2025

Fujian Medical University
Last update 22 Dec 2025
Overview
Tags
Neoplasms
Skin and Musculoskeletal Diseases
Nervous System Diseases
Small molecule drug
Proteolysis-targeting chimeras (PROTAC)
Exosomes
Disease domain score
A glimpse into the focused therapeutic areas
No Data
Technology Platform
Most used technologies in drug development
No Data
Targets
Most frequently developed targets
No Data
| Top 5 Drug Type | Count |
|---|---|
| Small molecule drug | 21 |
| Proteolysis-targeting chimeras (PROTAC) | 5 |
| Exosomes | 3 |
| Chemical drugs | 2 |
| Synthetic peptide | 2 |
Related
42
Drugs associated with Fujian Medical UniversityMechanism EDG6 modulators [+3] |
Active Org. |
Originator Org. |
Active Indication |
Drug Highest PhaseApproved |
First Approval Ctry. / Loc. United States |
First Approval Date21 Sep 2010 |
Target- |
Mechanism- |
Active Org. |
Originator Org. |
Inactive Indication |
Drug Highest PhaseApproved |
First Approval Ctry. / Loc. China |
First Approval Date01 Jan 1997 |
Target- |
Mechanism- |
Active Org. |
Originator Org.- |
Inactive Indication |
Drug Highest PhaseApproved |
First Approval Ctry. / Loc.- |
First Approval Date- |
330
Clinical Trials associated with Fujian Medical UniversityNCT07275034
A Virtual Reality-Based Horticultural Planting Program for Elderly With Subthreshold Depression:A Randomized Controlled Pilot Study
Subthreshold depression represents a state of psychological sub-health between normal individuals and clinical depression. It constitutes a high-risk stage for developing clinical depression and a critical phase for alleviating depressive symptoms. However, current research predominantly focuses on treating depression in the elderly, with insufficient attention to interventions for high-risk populations or limited to cross-sectional investigations of subthreshold depression risk factors. Therefore, prioritizing subthreshold depression in the elderly and implementing early interventions is essential. A 2024 article in The Lancet suggests that subthreshold depression treatment should prioritize psychotherapy and lifestyle adjustments over medication. Horticultural therapy, an interdisciplinary approach integrating horticulture, medicine, and psychology, demonstrates unique advantages over traditional medical treatments. In practical application, however, limited resources may hinder adequate support for horticultural activities, compromising activity quality, reducing therapeutic efficacy, and restricting the widespread adoption of horticultural therapy. Integrating VR technology with horticultural therapy can provide patients with more comprehensive, personalized, and effective mental health treatment plans, empowering them toward healthier, more positive lives. Therefore, the research team designed a VR horticultural intervention system for elderly individuals with subthreshold depression based on the Social Participation Competence Framework.
Start Date01 Feb 2026 |
Sponsor / Collaborator |
ChiCTR2500113743
Evidence-Based Development and Initial Application of Sleep Hygiene Education Content for Community-Dwelling Older Adults
Start Date02 Dec 2025 |
Sponsor / Collaborator |
NCT07210242
Clinical Study on Monitoring Cytomegalovirus (HCMV) Reactivation After Allogeneic Hematopoietic Stem Cell Transplantation Using High-Performance miRNA Quantification Technology (PSTM-qPCR)
Human cytomegalovirus (HCMV) infection is one of the most common and serious complications after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Standard monitoring uses HCMV DNA testing, but this method may not detect the virus early enough to guide timely treatment.
This multicenter observational study will evaluate a new high-performance microRNA (miRNA) detection technology (PSTM-qPCR) for monitoring HCMV infection in allo-HSCT patients. Approximately 300 patients and their donors will be enrolled across several major transplant centers in China. Blood samples will be collected before and after transplantation to test for both HCMV-miRNA and HCMV-DNA. The study will compare the sensitivity and timing of miRNA detection with conventional DNA testing and explore whether miRNA can serve as an early biomarker of infection and related complications.
The goal is to improve early diagnosis and management of HCMV infection, reduce infection-related complications, and ultimately improve survival outcomes in patients undergoing allo-HSCT.
This multicenter observational study will evaluate a new high-performance microRNA (miRNA) detection technology (PSTM-qPCR) for monitoring HCMV infection in allo-HSCT patients. Approximately 300 patients and their donors will be enrolled across several major transplant centers in China. Blood samples will be collected before and after transplantation to test for both HCMV-miRNA and HCMV-DNA. The study will compare the sensitivity and timing of miRNA detection with conventional DNA testing and explore whether miRNA can serve as an early biomarker of infection and related complications.
The goal is to improve early diagnosis and management of HCMV infection, reduce infection-related complications, and ultimately improve survival outcomes in patients undergoing allo-HSCT.
Start Date10 Oct 2025 |
Sponsor / Collaborator |
100 Clinical Results associated with Fujian Medical University
Login to view more data
0 Patents (Medical) associated with Fujian Medical University
Login to view more data
12,980
Literatures (Medical) associated with Fujian Medical University01 Apr 2026·BIOMATERIALS
“In situ endothelial modulation and transduction” strategy driven by biomimetic H2S delivery system for targeted repair of vascular injury
Article
Author: Yao, Peisen ; Zhang, Yibin ; Li, Shengnan ; Huang, Xiaofen ; Niu, Xuegang ; Kang, Dezhi ; Gao, Bin ; Lin, Yuanxiang
Vascular recanalization mediated by the interventional therapy can reduce cardio-cerebrovascular disease burden. However, its long-term outcomes are often undesired owing to (1) inevitable mechanical damage to the vasculature that triggers pathological remodeling, presented as local inflammation/oxidative stress, intimal hyperplasia, and delayed endothelial healing; and (2) long-term and frequent use of antiplatelet drugs, which increase bleeding risks. These challenges highlight the necessity for rapid vascular repair and minimizing dosing frequency, which are currently unmet due to the lack of a highly efficient delivery/therapy strategy. Herein, we formulated a damaged vascular endothelial-targeted hydrogen sulfide (H2S) nanomedicine, utilizing the endothelial cells (ECs) as a delivery destination rather than the traditional smooth muscle cells (SMCs) for overcoming drug delivery barriers. This drug targets the ECs, where it releases H2S in a sustained manner to promote endothelial regeneration and in situ transduce signaling from ECs for suppressing SMC-mediated intimal hyperplasia and reprogramming Mφ to inhibit local inflammation. A single dose of therapy achieved satisfactory vascular repair and safety within 28 days in the carotid artery injury model. This study provides a novel solution for vascular repair and advances the development of a drug delivery approach.
01 Feb 2026·IMMUNOLOGY LETTERS
Gut microbiota analysis revealed unique biomarkers in Ankylosing Spondylitis and Non-radiographic Axial Spondyloarthritis
Article
Author: Chang, Sijie ; Chen, Mingrong ; Zhang, Jinhua ; Niu, Peiguang
OBJECTIVES:
In this paper, the different characteristics of gut microbiota between Ankylosing Spondylitis (AS), Healthy Control (HC), and Non-radiographic Axial Spondyloarthritis (nr-axSpA) were studied. The AS-nr-axSpA differentiation model was constructed to identify patients with these two phenotypes and help doctors make accurate diagnosis.
METHODS:
Stool samples and blood samples of AS, nr-axSpA, and HC were collected from our hospital. Bacterial lipopolysaccharides and lipopolysaccharides-binding proteins in blood were detected by enzyme-linked immunosorbent assay (ELISA). The V3-V4 region of bacterial 16SrRNA was analyzed by MiSeq PE300 sequencing platform with high throughput. Software such as QIIME, R, Excel, etc. were used for statistical analysis of the data. Random Forest (RF) and Area Under Curve (AUC) methods were used to construct the AS-nr-axSpA differentiation model and identify relevant important markers. Set markers and use the receiver operating characteristic curve (ROC) to judge the accuracy of the model.
RESULTS:
We studied a total of 59 fecal and corresponding blood samples from 31 AS, 21 nr-axSpA, and 7 HC. There was a significant difference in intestinal α diversity between AS and nr-axSpA patients (Shannon index, P = 0.017). Compared to the nr-axSpA patient population, Streptococcus (P = 0.045), Actinomyces (P = 0.0028), Rothia (P = 0.042), and Oribacterium in the intestinal tract of AS patients P = 0.044) increased significantly. However, Dorea (P = 0.034) and Odoribacter (P = 0.043) were significantly reduced. The AS-nr-axSpA model was constructed using 18 factors including Actinomyces and Odoribacter. ROC analysis was performed on the model and an ROC curve was drawn, with an AUC of 0.78, which is moderate accurate.
CONCLUSIONS:
The gut microbiota of patients with AS differs from that of patients with nr-axSpA. The disturbance of gut microbiota may be one of the conditions for the progression of nr-axSpA to AS. The characteristics of gut microbiota and related bacterial products may serve as characteristic factors for differentiating the phenotypes of these two diseases. The AS-nr-axSpA model may help doctors distinguish patients with different phenotypes, but more robust prospective and standardized studies are needed to confirm these findings.
01 Feb 2026·JOURNAL OF COLLOID AND INTERFACE SCIENCE
Dual-activated nanomotors as a theranostic platform for photoacoustic imaging-guided combination cancer therapy
Article
Author: Li, Xiao ; Pan, Jianqiong ; Hong, Shanni ; Xu, Guizhen ; Peng, Yixian ; Feng, Yuanfang ; Lin, Xiahui ; Meng, Qingao
High concentrations of nitric oxide (NO) can induce tumor cell death. However, its limited biological half-life and off-target effects restrict its clinical application. Herein, a dual-triggered NO-releasing nanomotor with an asymmetric bowl structure was designed, which is composed of tightly assembled gold nanoparticles and loaded with the NO donor N,N'-di-sec-butyl-N,N'-di-nitroso-1,4-phenylenediamine (BNN6). The special bowl-like structure provided a self-thermophoretic propulsion for nanomotors under near-infrared (NIR) light irradiation, enhancing their diffusion in tumor tissue. Furthermore, while achieving NO release, photoacoustic (PA) imaging-guided photothermal therapy (PTT) has also been realized. Additionally, with the assistance of ultrasound (US) perturbation, the nanomotor synergizes with NIR light to further promote the penetration capacity of the nanomotor in the dense tumor tissue. In addition, the special structure enhances the ultrasonic cavitation effect, which induces reactive oxygen species (ROS) production, thereby resulting in cell death by oxidative stress and achieving sonodynamic therapy (SDT). Notably, ROS and NO results in the formation of reactive nitrogen species (RNS) with higher toxicity, which further enhances antitumor efficacy. This strategy integrates gas therapy, PTT, and SDT via NIR/US dual-activated nanomotors, combining RNS with local hyperthermia for enhanced tumor treatment, offers an inspiration for developing novel cancer therapeutics.
3
News (Medical) associated with Fujian Medical University14 Aug 2025
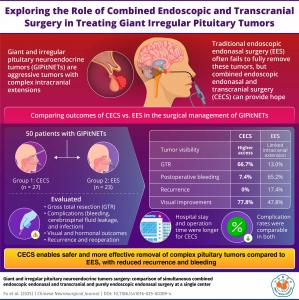
Two-team surgical approach removes more complex pituitary tumors, reduces bleeding risk, and lowers recurrence compared to traditional endonasal surgery
BEIJING, BEIJING, CHINA, August 14, 2025 /
EINPresswire.com
/ -- The pituitary gland, located at the base of the brain, secretes hormones that regulate vital body functions and control the activity of other hormone-secreting glands. Pituitary neuroendocrine tumors (PitNETs) are abnormal growths in this gland. In recent years, endoscopic endonasal surgery (EES), a minimally invasive technique, has become a widely used method for treating these tumors. In this approach, an endoscope is inserted through the nasal passages and sinuses—a route referred to as endonasal.
However, giant and irregular pituitary neuroendocrine tumors (GIPitNETs) pose a significantly greater challenge. These tumors are typically larger than 4 cm and often extend beyond the sella, the bony structure that houses the pituitary gland. They may grow upwards into the cranial cavity, the space within the skull that houses the brain. This extension can render the tumor inaccessible or invisible through the standard EES approach.
An alternative and innovative method used in such cases is the combined endoscopic endonasal and transcranial surgery (CECS). In this technique, one surgical team performs the EES approach while another team simultaneously carries out transcranial surgery, which involves accessing the tumor by creating small openings in the skull.
To analyze and compare the efficacy and complications of CECS and EES for GIPitNETs treatment, a team of researchers from China conducted a retrospective observational single-center cohort study. The study led by Dr. Changzhen Jiang and Dr. Xiaorong Yan from the Neurosurgery Research Institute, Fujian Medical University, China, was published in the
Chinese Neurosurgical Journal
and made available online on February 03, 2025. “We wanted to define the limitations and benefits of the two surgical procedures in the management of GIPitNETs,” says Dr. Jiang while explaining the aim of the study.
The research included 50 patients who underwent either EES or CECS between March 2018 and May 2023 at The First Affiliated Hospital of Fujian Medical University. All patients had tumors larger than 4 cm with significant intracranial extension. Endocrine tests were conducted before and after surgery to assess hormone levels. Magnetic resonance imaging (MRI) was used to evaluate tumor size and post-surgical outcomes. Researchers also analyzed hospital records for symptoms and complications, and ophthalmologists assessed visual function before and after surgery.
27 out of the 50 patients enrolled for this study were treated by CECS, and EES was performed on the remaining 23 patients. The researchers compared the obtained data using statistical analysis.
The results revealed a higher rate of gross total tumor removal (GTR) in the CECS group. GTR was achieved in 66% of patients in the CECS group, compared to just 13% in the EES group. Postoperative bleeding, a common and serious complication, was more prevalent in the EES group—65.2% compared to 7.4% in the CECS group. Additionally, all four cases of tumor recurrence due to residual tumor were reported in the EES group, suggesting that incomplete removal in EES may increase the risk of recurrence.
Interestingly, even though CECS achieved better tumor removal, visual outcomes were similar in both groups. “Partial tumor removal can also alleviate the pressure on optic nerves and lead to symptom relief. Thus, visual symptom improvement is possible after undergoing EES,” explains Dr. Yan.
However, CECS is not without its limitations. “The CECS technique requires a longer operation time and has greater surgical trauma with similar postoperative infection rates, compared to EES,” notes Dr. Jiang. Patients in the CECS group also had longer hospital stays. Despite the increased invasiveness of CECS, postoperative infection rates were comparable to those of the less invasive EES, indicating that CECS remains a safe option when performed carefully by experienced surgical teams.
Overall, the findings suggest that CECS may offer significant advantages in treating GIPitNETs, particularly when the tumor’s size or shape makes it inaccessible by EES alone. The improved GTR rate and lower complication rate point to CECS as a more effective approach for complex cases, despite the longer operation and recovery times.
The research team plans to further investigate the long-term efficacy and safety of CECS. They aim to conduct follow-up studies over extended periods and hope to analyze more patient data through large-scale multicenter collaborations. With continuous improvements in surgical techniques, approaches like CECS may help make the treatment of complex tumors like GIPitNETs safer and more successful.
***
Reference
Title of original paper: Giant and irregular pituitary neuroendocrine tumors surgery: comparison of simultaneous combined endoscopic endonasal and transcranial and purely endoscopic endonasal surgery at a single center
Journal: Chinese Neurosurgical Journal
DOI: 10.1186/s41016-025-00389-4
About Fujian Medical University
Fujian Medical University (FJMU), established in 1937, is a leading medical research university in China. It has over 20,000 full-time students and a strong faculty, more than half of whom hold doctorates or other advanced degrees. FJMU drives innovation in molecular medicine, oncology, and public health. The university hosts several national and provincial research platforms, including key laboratories and clinical trial centers. FJMU’s emphasis on translational medicine and interdisciplinary collaboration fosters cutting-edge discoveries. FJMU also participates in global research networks and offers English-medium postgraduate programs, attracting international scholars and supporting China’s broader scientific and medical advancement.
Website:
https://oec.fjmu.edu.cn/en/
About Dr. Xiorong Yan, from Fujian Medical University
Dr. Xiaorong Yan is a neurosurgeon at the Neurosurgery Research Institute, First Affiliated Hospital of Fujian Medical University in Fuzhou, China. She holds an MD in Neurosurgery from Shandong Medical University. Her work mainly focuses on endoscopic surgery for pituitary adenomas and other brain lesions. She co-led the recent comparison study of combined endoscopic endonasal‑transcranial surgery versus purely endonasal surgery for giant pituitary tumors, contributing to improved surgical strategies and patient outcomes. Dr. Yan has also contributed to other minimally invasive neurosurgical techniques, publishing clinical studies on spine and brain‑tumor procedures. She has over 20 published papers.
Funding information
This work was sponsored by Joint Funds for Innovation of Science and Technology, Fujian Province (No. 2021Y9089), University-Industry Research Joint Innovation Project of Science and Technology, Fujian Province (No. 2023Y4018), Fujian Province Finance Project (No. BPB-2022YXR), scientific research, Fujian Medical University (grant number 2022QH1098).
Yi Lu
Chinese Neurosurgical Journal
+861059978478 ext.
- luyi617@sina.cn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability
for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this
article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Clinical Result
02 Nov 2023
Major depressive disorder (MDD) is a widespread mental health condition that for many is disabling. It has long been appreciated that MDD has genetic as well as environmental influences. In a new study researchers identify a gene that interacted with stress to mediate aspects of treatment-resistant MDD in an animal model.
Major depressive disorder (MDD) is a widespread mental health condition that for many is disabling. It has long been appreciated that MDD has genetic as well as environmental influences. In a new study in Biological Psychiatry, published by Elsevier, researchers identify a gene that interacted with stress to mediate aspects of treatment-resistant MDD in an animal model.
Jing Zhang, PhD, at Fujian Medical University and senior author of the study, said, "Emerging evidence suggests that MDD is a consequence of the co-work of genetic risks and environmental factors, so it is crucial to explore how stress exposure and risk genes co-contribute to the pathogenesis of MDD."
To do that, the authors used a mouse model of stress-induced depression called chronic social defeat stress (CSDS) in which mice are exposed to aggressor mice daily for two weeks. They focused on a gene called LHPP, which interacts with other signaling molecules at neuronal synapses. Increased expression of LHPP in the stressed mice aggravated the depression-like behaviors by decreasing expression of BDNF and PSD95 by dephosphorylating two protein kinases, CaMKIIα and ERK, under stress exposure.
Dr. Zhang noted, "Interestingly, LHPP mutations (E56K, S57L) in humans can enhance CaMKIIα/ERK-BDNF/PSD95 signaling, which suggests that carrying LHPP mutations may have an antidepressant effect in the population."
MDD is an extremely heterogeneous condition. Differences in the types of depression experienced by people influence the way they respond to treatment. A large subgroup of people with depression fail to respond to standard antidepressant medications and have "treatment-resistant" symptoms of depression. These patients often respond to different medications, such as ketamine or esketamine, or to electroconvulsive therapy. Notably, esketamine markedly alleviated LHPP-induced depression-like behaviors, whereas the traditional drug fluoxetine did not, suggesting that the mechanism might underlie some types of treatment-resistant depression.
John Krystal, MD, Editor of Biological Psychiatry, said of the work, "We have limited understanding of the neurobiology of treatment-resistant forms of depression. This study identifies a depression risk mechanism for stress-related behaviors that fail to respond to a standard antidepressant but respond well to ketamine. This may suggest that the risk mechanisms associated with the LHPP gene shed light on the poorly understood biology of treatment-resistant forms of depression."
Dr. Zhang added, "Together, our findings identify LHPP as an essential player driving stress-induced depression, implying targeting LHPP as an effective strategy in MDD therapeutics in the future."
08 Aug 2023
MONDAY, Aug. 7, 2023 -- Gadolinium-enhanced magnetic resonance imaging (MRI) of the inner ear can help differentiate Meniere disease (MD) from vestibular migraine (VM), according to a study published online June 26 in
The Laryngoscope
.
Heng Xiao, from Fujian Medical University in Fuzhou, China, and colleagues examined predictive factors for MD and VM in 87 patients (50 MD and 37 VM) who underwent intratympanic injection of gadolinium and then MRI of the inner ear 24 hours later.
The researchers found that 92 percent of the patients in the MD group developed endolymphatic hydrops, while only two patients (5.4 percent) had positive results in the VM group. The incidence of migraine was 14 and 67.7 percent in the MD and VM groups. The greater the sum of the maximum slow phase velocity of the ipsilateral (SSPVI) ear, the higher the risk for VM occurrence in multivariate regression of the two groups of patients. There was a positive correlation observed for the incidence of carsickness with incidence of VM, while a negative correlation was seen for asymmetric hearing loss (AHL) with VM diagnosis.
"Although gadolinium-enhanced MRI of the inner ear is helpful in the differential diagnosis of VM and MD, less invasive differential symptomology, including carsickness, decrease in AHL in audiology examination and increase in SSPVI of the ipsilateral ear in vestibular function examinations may also serve as diagnostic predictors of VM," the authors write.
Abstract/Full Text
Posted August 2023
Clinical Result
100 Deals associated with Fujian Medical University
Login to view more data
100 Translational Medicine associated with Fujian Medical University
Login to view more data
Corporation Tree
Boost your research with our corporation tree data.
login
or

Pipeline
Pipeline Snapshot as of 18 Feb 2026
The statistics for drugs in the Pipeline is the current organization and its subsidiaries are counted as organizations,Early Phase 1 is incorporated into Phase 1, Phase 1/2 is incorporated into phase 2, and phase 2/3 is incorporated into phase 3
Discovery
3
36
Preclinical
Phase 1
2
6
Other
Login to view more data
Current Projects
| Drug(Targets) | Indications | Global Highest Phase |
|---|---|---|
Chimeric Natural Killer Receptor-Universal T Cells(Fujian Medical University) | Steroid Refractory Graft Versus Host Disease More | Phase 1 |
Koumine Hydrochloride | Rheumatoid Arthritis More | Phase 1 |
Exosome of Mesenchymal Stem Cells(Fujian Medical University) | Multiple Organ Failure More | Clinical |
Compound CP1(FMU) ( DAPK1 ) | Neurodegenerative Diseases More | Preclinical |
tRF-23-Z87HFK8SDZ ( IRS-1 ) | Pancreatic Cancer More | Preclinical |
Login to view more data
Deal
Boost your decision using our deal data.
login
or

Translational Medicine
Boost your research with our translational medicine data.
login
or

Profit
Explore the financial positions of over 360K organizations with Synapse.
login
or

Grant & Funding(NIH)
Access more than 2 million grant and funding information to elevate your research journey.
login
or

Investment
Gain insights on the latest company investments from start-ups to established corporations.
login
or

Financing
Unearth financing trends to validate and advance investment opportunities.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free



