Request Demo
Last update 11 Feb 2026

Opiant Pharmaceuticals, Inc.
Last update 11 Feb 2026
Overview
Tags
Other Diseases
Small molecule drug
Disease domain score
A glimpse into the focused therapeutic areas
No Data
Technology Platform
Most used technologies in drug development
No Data
Targets
Most frequently developed targets
No Data
| Top 5 Drug Type | Count |
|---|---|
| Small molecule drug | 3 |
| Top 5 Target | Count |
|---|---|
| CB1R(Cannabinoid CB1 receptor) | 1 |
| Opioid receptors | 1 |
Related
3
Drugs associated with Opiant Pharmaceuticals, Inc.Target |
Mechanism Opioid receptors antagonists |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication- |
Drug Highest PhasePhase 2 |
First Approval Ctry. / Loc.- |
First Approval Date- |
Target- |
Mechanism- |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication- |
Drug Highest PhaseEarly Phase 1 |
First Approval Ctry. / Loc.- |
First Approval Date- |
Target |
Mechanism CB1 antagonists |
Active Org. |
Originator Org. |
Active Indication |
Inactive Indication |
Drug Highest PhasePreclinical |
First Approval Ctry. / Loc.- |
First Approval Date- |
5
Clinical Trials associated with Opiant Pharmaceuticals, Inc.NCT05219669
An Open-Label, Three-Period, Three-Treatment, Six-Sequence, Randomized Crossover Study of the Pharmacokinetics of Intranasal Nalmefene in Healthy Volunteers Using Three Dosing Regimens
This study is to compare the pharmacokinetics (how the body absorbs, breaks down and eliminates drug from your body) of nalmefene when given as a single dose intranasally (IN;into the nose), as a single dose in each nostril and as two doses in one nostrils; and to evaluate the safety and tolerability of nalmefene IN.
Start Date01 Sep 2021 |
Sponsor / Collaborator |
NCT04828005
A Two-part Open Label Study of the Pharmacodynamic Effects of Intranasal Nalmefene Compared to Intranasal Naloxone in Healthy Volunteers Under Steady State Opioid Agonism
This study is to determine the pharmacodynamics (the effects of the drug and mechanisms of their action within the body) of Nalmefene when given intranasally (IN; into the nose) compared to intranasal naloxone when given to healthy volunteers under steady state opioid agonism.
Start Date30 Mar 2021 |
Sponsor / Collaborator |
NCT04759768
Two-Period, Two-Treatment, Randomized Crossover Study of the Pharmacokinetics of Nalmefene by Intranasal and Intramuscular Administration in Healthy Volunteers
This study is to determine the pharmacokinetics (how the body absorbs, breaks down and eliminates drug from your body) of nalmefene when given intranasally (IN;into the nose) compared to a dose of nalmefene when given intramuscularly (IM; into the muscle); to compare the blood levels of nalmefene when given IN to nalmefene when given IM; and to evaluate the safety and tolerability of nalmefene IN.
Start Date08 Feb 2021 |
Sponsor / Collaborator |
100 Clinical Results associated with Opiant Pharmaceuticals, Inc.
Login to view more data
0 Patents (Medical) associated with Opiant Pharmaceuticals, Inc.
Login to view more data
6
Literatures (Medical) associated with Opiant Pharmaceuticals, Inc.01 Feb 2020·Journal of neural transmission (Vienna, Austria : 1996)
Cannabinoid1 (CB-1) receptor antagonists: a molecular approach to treating acute cannabinoid overdose
Review
Author: Crystal, Roger ; Skolnick, Phil
Abstract:
The legalization of cannabis for both recreational and medical use in the USA has resulted in a dramatic increase in the number of emergency department visits and hospital admissions for acute cannabinoid overdose (also referred to as cannabis intoxication and cannabis poisoning). Both “edibles” (often sold as brownies, cookies, and candies) containing large amounts of Δ9-tetrahydrocannabinol and synthetic cannabinoids (many possessing higher potencies and efficacies than Δ9-tetrahydrocannabinol) are responsible for a disproportionate number of emergency department visits relative to smoked cannabis. Symptoms of acute cannabinoid overdose range from extreme lethargy, ataxia, and generalized psychomotor impairment to feelings of panic and anxiety, agitation, hallucinations, and psychosis. Treatment of acute cannabinoid overdose is currently supportive and symptom driven. Converging lines of evidence indicating many of the symptoms which can precipitate an emergency department visit are mediated through activation of cannabinoid1 receptors. Here, we review the evidence that cannabinoid1 receptor antagonists, originally developed for indications ranging from obesity to smoking cessation and schizophrenia, provide a molecular approach to treating acute cannabinoid overdose.
01 Dec 2019·European neuropsychopharmacology : the journal of the European College of NeuropsychopharmacologyQ3 · MEDICINE
Be positive about negatives–recommendations for the publication of negative (or null) results
Q3 · MEDICINE
Review
Author: Bespalov, Anton ; Skolnick, Phil ; Steckler, Thomas
Both positive and negative (null or neutral) results are essential for the progress of science and its self-correcting nature. However, there is general reluctance to publish negative results, and this may be due a range of factors (e.g., the widely held perception that negative results are more difficult to publish, the preference to publish positive findings that are more likely to generate citations and funding for additional research). It is particularly challenging to disclose negative results that are not consistent with previously published positive data, especially if the initial publication appeared in a high impact journal. Ideally, there should be both incentives and support to reduce the costs associated with investing efforts into preparing publications with negative results. We describe here a set of criteria that can help scientists, reviewers and editors to publish technically sound, scientifically high-impact negative (or null) results originating from rigorously designed and executed studies. Proposed criteria emphasize the importance of collaborative efforts and communication among scientists (also including the authors of original publications with positive results).
01 Aug 2019·BMJ Open
Treating gambling disorder with as needed administration of intranasal naloxone: a pilot study to evaluate acceptability, feasibility and outcomes
Article
Author: Mäkelä, Niklas ; Salonen, Anne H ; Alho, Hannu ; Scheinin, Mika ; Castrén, Sari ; Crystal, Roger ; Haikola, Janne
Background and aim:
There is growing interest in the use of medication-assisted treatments for gambling disorder (GD). Opioid receptor antagonists are hypothesised to blunt the craving associated with gambling. This study was designed to assess the feasibility of using an intranasal naloxone spray to treat GD.
Design:
An 8-week, open-label, uncontrolled pilot study.
Setting:
A single study site in the capital region of Finland.
Subjects:
Twenty problem gamblers (nine men) were randomised into two groups. Group A (n=10) took one dose into one nostril (2 mg naloxone), as needed, with a maximum of 4 doses/day (max. 8 mg/day). Group B (n=10) took one dose into each nostril (4 mg naloxone) as needed, with a maximum of 4 doses/day (max. 16 mg/day).
Intervention:
Naloxone hydrochloride nasal spray.
Measures:
Acceptability and feasibility of the intervention were assessed. Use of study medication, adverse events, gambling frequency and gambling expenditure were recorded in a mobile diary. Problem gambling: South Oaks Gambling Screen (SOGS), depressive symptoms: Beck Depression Inventory (BDI) and alcohol use: Alcohol Use Disorders Identification Test were recorded.
Results:
Study completion rate was 90%. Acceptability and feasibility scores were high. Group B used intranasal naloxone more frequently than group A, and consequently used more naloxone. No serious adverse events were reported. The postintervention SOGS scores were lower (median=4 (IQR=3.75) versus preintervention scores (median=12 (IQR=4.75)). Depressive symptoms were reduced during the trial (preintervention BDI median=9, IQR=9 vs postintervention BDI median=6, IQR=6).
Conclusions:
The acceptability and feasibility of using intranasal naloxone were high, and no serious adverse events were reported. Preliminary results suggest mixed results in terms of gambling behaviour (ie, reduced frequency but not expenditure) and decreased depressive symptoms.
Trial registration number:
EudraCT2016-001828-56
72
News (Medical) associated with Opiant Pharmaceuticals, Inc.23 Dec 2025
Igor Kutyaev/
iStock
The banker allegedly shared details of a series of multibillion-dollar buyouts by companies including AbbVie, GSK and Pfizer.
The U.S. Attorney’s Office for the District of New Jersey has
charged
six people with running an insider trading scheme that allegedly made $41 million off trades informed by pharma M&A secrets leaked by an investment banker.
The banker, Gyunho Justin Kim, allegedly
shared
information on nine buyouts between 2020 and 2023. The sequence ran from Gilead Sciences’
$21 billion takeover
of Immunomedics to AbbVie’s
$10.1 billion buyout
of ImmunoGen. In between, Kim allegedly shared material nonpublic information on other deals struck by companies including Amgen, Biogen and GSK.
Fierce Biotech
reports that Kim worked in Citigroup’s San Francisco office.
Prosecutors claim Pfizer’s $5.4 billion Global Blood Therapeutics acquisition was the most profitable trade. According to the court filing, the investment bank where Kim worked represented a company that tried to buy GBT. Kim allegedly met his friend Saad Shoukat, a co-conspirator and the claimed conduit for information to other people in the scheme, around the time that the company was looking into GBT.
Saad Shoukat and six other people allegedly bought GBT stock in the months before the first media reports of takeover interest. With the reports boosting GBT’s stock, the individuals allegedly sold their stakes and collectively made illegal gains of more than $20 million.
The alleged insider trading activity is one of three overlapping securities fraud schemes presented in the court filing. The other two schemes involve market manipulation. Saad Shoukat and his brother Arham Shoukat are accused of manipulating Olema Pharmaceuticals’ stock price. Another brother, Shahwaiz Shoukat, allegedly worked with Saad and Arham to manipulate Opiant Pharmaceuticals’ share price.
Saad Shoukat allegedly bought Opiant stock after Kim told him a company was seeking to acquire the biotech, which was developing an opioid overdose treatment. The potential buyout stalled. Stuck with his stock purchases, Saad Shoukat allegedly worked with his brothers to set up a fake Opiant website and email addresses and send a fake press release announcing a purported deal involving the company.
With the press release going out on Cision PR Newswire, Opiant’s share price jumped 29% in the wake of the fake news. The
newswire
and
Opiant
later told journalists and investors to disregard the fake press release. Meanwhile, Opiant’s stock had jumped 29% and the Shoukats had sold shares during the price spike, according to the lawsuit.
Two of the brothers, Saad and Arham, allegedly manipulated Olema’s share price in 2021. The lawsuit claims the brothers invested in Olema, without any input from Kim, and used hacking techniques such as spoofing and social engineering to access confidential information. The information showed Olema’s breast cancer drug candidate OP-1250 was less effective than they had hoped, according to the lawsuit.
Saad and Arham allegedly falsified OP-1250 data, inflating the molecule’s efficacy, and made it look like an official Olema publication. Olema
issued
a statement about falsified information circulating on social media on Nov. 29, 2021. The biotech’s stock closed up that day. The Shoukats allegedly sold their shares during the price bump. On Nov. 30, 2021, the
release
of the real data sparked a drop in Olema’s stock.
AcquisitionPatent Infringement
09 Aug 2024
Purdue, after playing major role in US opioid epidemic, wins FDA nod for overdose-reversal injection
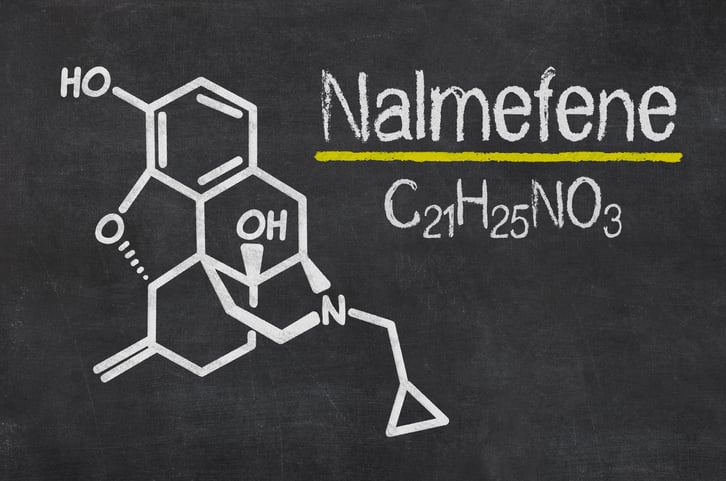
Prior to Zurnai’s approval, Purdue had already been supplying nalmefene hydrochloride injection to emergency departments across the U.S. at no profit.
As Purdue Pharma attempts to restore its image following its role in the U.S. opioid crisis, the Connecticut-based company has scored a win with a green light for a new overdose rescue treatment.This week, the FDA approved Purdue’s nalmefene injection Zurnai to treat known or suspected opioid overdoses in adults and kids ages 12 and older. The drug, which comes in a 1.5 mg autoinjector, can be used to treat overdoses from both prescription opioids and synthetic opioids such as fentanyl, Purdue said in a release.Zurnai is meant for immediate use in emergency settings and is not a substitute for medical care, the company added.The medicine’s autoinjector has been designed for ease of use by “anyone in the community,” from healthcare professionals and first responders to bystanders, caregivers and family members, Purdue said. The active ingredient in Zurnai, nalmefene, is the longest-acting opioid antagonist approved for overdose reversal, according to Purdue’s release. Prior to Zurnai’s approval, Purdue had already been supplying nalmefene hydrochloride injection to emergency departments across the U.S. at no profit.Purdue’s nalmefene autoinjector nod comes a little more than a year after Indivior’s Opvee became the first approved nalmefene nasal spray. Indivior got its hands on the opioid overdose reversal agent through its $145 million buyout of Opiant in late 2022. Like Zurnai, Opvee is approved for use in people ages 12 and up.Zurnai was approved based on safety and pharmacokinetic studies, plus a study in healthy people who use opioids recreationally, to see how quickly the product works, the FDA said in its own press release.And while Zurnai certainly may have a role to play in preventing deaths from overdoses, the FDA warned of the unexpected consequences of using the med on people who are opioid dependent—an issue that has raised concerns about overdose reversal agents writ large.If patients who are opioid dependent are given Zurnai, they may go into opioid withdrawal. In turn, people addicted to opioids who recover from an overdose may leave the care of doctors or first responders if their symptoms are ignored, and they’re at risk of using opioids again in an effort to curb withdrawal symptoms, STAT News reports.Drug overdoses continue to pose a major health threat in the U.S., and more than 107,000 fatal overdoses were reported last year, the FDA said. Those deaths were primarily driven by synthetic opioids like illicit fentanyl, the agency warned.The approval comes less than two months after the Supreme Court blocked a multi-billion-dollar bankruptcy agreement for Purdue Pharma and its founding family, the Sacklers.Under the proposed deal, the Sacklers were set to return up to $6 billion to Purdue’s bankruptcy estate in exchange for immunity from civil lawsuits tied to the nationwide opioid crisis. Over the years, Purdue Pharma and the Sacklers have faced countless lawsuits and a swarm of public criticism for the role that their misleading OxyContin marketing played in the U.S. opioid crisis.Back in 2020, Purdue Pharma agreed to plead guilty in federal court to a three-count felony charge tied to a “dual-object conspiracy to defraud the United States and to violate the Food, Drug, and Cosmetics Act.” The company also pleaded guilty to two counts of conspiracy to violate the Federal Anti-Kickback Statute.As part of the decision, Purdue was slapped with a $3.5 billion criminal fine, plus an additional $2 billion criminal forfeiture—a punishment the U.S. Justice Department at the time called the “largest penalty ever levied against a pharmaceutical manufacturer.”Perhaps most notably, under the resolution, Purdue was forced to cease to operate in its original form and emerge from bankruptcy as a public benefit company (PBC) to “function entirely in the public interest,” the Justice Department said.
Drug ApprovalPatent Infringement
10 Jul 2024
Indivior has cut its sales estimate for its top-selling product Sublocade. It also is grounding schizophrenia drug Perseris and laying off 130 staffers.
Just seven weeks after projecting an 18% revenue boost for this year, Indivior has slashed its estimate to an 8% increase, citing competitive pressures for its opioid addiction treatment Sublocade and “payor dynamics” for its schizophrenia drug Perseris.
The Richmond, Virginia-based company will discontinue sales of Perseris, despite it achieving a sales increase of 50% last year to $42 million. The grounding of Perseris will save the company $50 million, Indivior said, as it will lay off approximately 130 staffers.
The company also revealed it will pay $85 million to resolve an antitrust case which was set to go to trial on July 15. It is another in a series of settlements Indivior has paid over the last several years to resolve antitrust and aggressive marketing claims.
As for the company’s top-selling product Sublocade, Indivior has dropped its 2024 sales projection from $820 million to $880 million to a window of $765 million to $805 million. Last year, Sublocade generated $630 million in revenue for a 54% year-over-year increase.
Accordingly, Indivior has sliced its overall 2024 revenue estimate from $1.24 billion to $1.33 billion to a new range of $1.15 billion to $1.22 billion.
With Tuesday’s business update, Indivior’s share price has dropped by 33%.
“We are taking decisive action that we believe is in the best interest of shareholders,” Indivior CEO Mark Crowley said in a release.
Crowley added that “increased payor management” made Perseris’ “future no longer financially viable.”
Meanwhile, new competition from Camurus’ extended-release opioid use disorder treatment Brixadi has cut into the sales of Sublocade. Another factor negatively affecting sales has been the “elimination of COVID emergency measures related to automatic Medicaid coverage renewals,” Crowley said.
The company also is counting on its new opioid overdose medicine, Opvee, a nasal spray which was approved 14 months ago after holding its own against Emergent’s market leader naloxone in a head-to-head study. Indivior gained Opvee in a 2023 acquisition of Opiant for $145 million.
Acquisition
100 Deals associated with Opiant Pharmaceuticals, Inc.
Login to view more data
100 Translational Medicine associated with Opiant Pharmaceuticals, Inc.
Login to view more data
Corporation Tree
Boost your research with our corporation tree data.
login
or

Pipeline
Pipeline Snapshot as of 07 Mar 2026
The statistics for drugs in the Pipeline is the current organization and its subsidiaries are counted as organizations,Early Phase 1 is incorporated into Phase 1, Phase 1/2 is incorporated into phase 2, and phase 2/3 is incorporated into phase 3
Preclinical
1
1
Early Phase 1
Phase 2
1
3
Other
Login to view more data
Current Projects
| Drug(Targets) | Indications | Global Highest Phase |
|---|---|---|
Intranasal nalmefene(Opiant Pharmaceuticals, Inc.) ( Opioid receptors ) | Opioid-Related Disorders More | Phase 2 |
OPT004 | Drug Overdose More | Early Phase 1 |
Drinabant ( CB1R ) | Obesity More | Discontinued |
OPNT-005 | Heroin Dependence More | Pending |
Naltrexone ( Opioid receptors ) | Alcohol Use Disorder More | Pending |
Login to view more data
Deal
Boost your decision using our deal data.
login
or

Translational Medicine
Boost your research with our translational medicine data.
login
or

Profit
Explore the financial positions of over 360K organizations with Synapse.
login
or

Grant & Funding(NIH)
Access more than 2 million grant and funding information to elevate your research journey.
login
or

Investment
Gain insights on the latest company investments from start-ups to established corporations.
login
or

Financing
Unearth financing trends to validate and advance investment opportunities.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free
