Request Demo
Last update 29 Aug 2025
Samyang Biopharmaceuticals Corp.
Last update 29 Aug 2025
Overview
Basic Info
Introduction Samyang Biopharmaceuticals Corporation originated as the pharmaceutical business unit of Samyang Corporation in 1995. In 2011, it separated from Samyang Corporation and became Samyang Biopharm. Subsequently, in 2021, it merged with Samyang Holdings, the holding company, with the goal of enhancing mid and long-term corporate values and establishing itself as a global biopharmaceutical company. The company's headquarters are situated in South Korea.
Samyang Biopharmaceuticals focuses its business activities in the areas of medical devices, pharmaceutical products, and gene therapeutics. |
Related
32
Clinical Trials associated with Samyang Biopharmaceuticals Corp.NCT06961448
A Prospective, Multi-center, Evaluator-blinded, Randomized, Parallel-controlled, Superiority Clinical Trial to Evaluate the Safety and Efficacy of Polycaprolactone Microsphere Fillers for Correction of Moderate to Severe Nasolabial Folds
The goal of this clinical trial is to evaluate the safety and efficacy of polycaprolactone microsphere filler (Lafullen) for the correction of moderate to severe nasolabial folds in adults aged 18 and above, regardless of gender.
The main questions it aims to answer are:
* Does Lafullen improve wrinkle severity in the nasolabial fold area at 48 weeks post-injection (WSRS responder rate)?
* Is Lafullen superior to the control product (Restylane, a hyaluronic acid filler) in terms of long-term wrinkle correction and safety? Researchers will compare Lafullen (polycaprolactone filler) with Restylane (modified sodium hyaluronate gel) to see if Lafullen provides superior wrinkle correction at 12 months post-injection.
Participants will:
* Receive either Lafullen or Restylane via injection in the nasolabial fold area.
* Optionally receive a touch-up injection at 4 weeks after the first treatment if needed.
* Undergo regular follow-up visits at 4, 12, 24, 36, and 48 weeks post-treatment.
* Subjects in the Lafullen group will continue follow-up up to 72 weeks to assess long-term efficacy and safety.
* Be assessed through clinical photography, pain scale (VAS), satisfaction questionnaires, and safety evaluations (e.g., AEs, vital signs, lab tests).
The main questions it aims to answer are:
* Does Lafullen improve wrinkle severity in the nasolabial fold area at 48 weeks post-injection (WSRS responder rate)?
* Is Lafullen superior to the control product (Restylane, a hyaluronic acid filler) in terms of long-term wrinkle correction and safety? Researchers will compare Lafullen (polycaprolactone filler) with Restylane (modified sodium hyaluronate gel) to see if Lafullen provides superior wrinkle correction at 12 months post-injection.
Participants will:
* Receive either Lafullen or Restylane via injection in the nasolabial fold area.
* Optionally receive a touch-up injection at 4 weeks after the first treatment if needed.
* Undergo regular follow-up visits at 4, 12, 24, 36, and 48 weeks post-treatment.
* Subjects in the Lafullen group will continue follow-up up to 72 weeks to assess long-term efficacy and safety.
* Be assessed through clinical photography, pain scale (VAS), satisfaction questionnaires, and safety evaluations (e.g., AEs, vital signs, lab tests).
Start Date01 May 2025 |
Sponsor / Collaborator |
NCT06402058
A Randomized, Subject and Evaluator Blinded, Matched Pairs, Pilot Study to Evaluate the Safety and Efficacy of DMSB01 According to the Number of Injections in Subjects With Crow's Feet Lines Compared to Control Device
The objective of this Pilot Study is to verify the safety and efficacy of DMSB01 in the temporary improvement of Crow's Feet Lines
Start Date10 Jun 2024 |
Sponsor / Collaborator |
NCT06295172
A Randomized, Evaluator-blind, Matched Pairs, Prospective, Non Inferiority, Confirmatory Study to Compare the Safety and Efficacy Between Lafullen15 and Lafullen in the Correction of Nasolabial Folds
The objective of this clinical trial is to verify the safety and efficacy of Lafullen15 in the temporary improvement of nasolabial Folds
Start Date27 May 2024 |
Sponsor / Collaborator |
100 Clinical Results associated with Samyang Biopharmaceuticals Corp.
Login to view more data
0 Patents (Medical) associated with Samyang Biopharmaceuticals Corp.
Login to view more data
22
Literatures (Medical) associated with Samyang Biopharmaceuticals Corp.31 Jul 2023·Gland surgery
The novel use and feasibility of hemostatic oxidized regenerated cellulose agent (SurgiGuard®): multicenter retrospective study.
Article
Author: Kim, Kyung Sik ; Kim, Hyun Koo ; Rho, Seoung Yoon ; Choi, Sae Byeol ; Hong, Jae-Young ; Yun, Sangchul ; Lee, Maria ; Jin, Miryung ; Park, Jong-Hwa ; Park, Jeong-Ik
Background:
SurgiGuard® is an absorbent hemostatic agent based on oxidized regenerated cellulose. The efficacy, effects and safety of SurgiGuard® are equivalent to existing hemostatic agents in animal experiments. This study was designed to confirm that the use of SurgiGuard® alone is effective, safe and feasible compared to combination with other hemostatic methods.
Methods:
We retrospectively reviewed clinical data from 12 surgery departments in seven tertiary centers in South Korea nationwide. All surgeries were performed between January and December 2018.
Results:
A total of 807 patients were enrolled; 447 patients (55.4%) had comorbidities. The rate of major surgery (operative time ≥4 hours) was 44% (n=355 patients). Regarding the type of SurgiGuard® used in surgery, more than 70% of minor surgeries used non-woven types. In major surgery, more than five SurgiGuards® were used in 7.3% (26 patients), and the proportion of co-usage (with four other hemostatic products) was 19.7% (70 patients). The effectiveness score was higher when SurgiGuard® was used alone in both major (5.3±0.5 vs. 5.1±0.6, P=0.048) and minor surgery (5.4±0.6 vs. 5.2±0.4, P<0.001). Seven patients had immediate re-bleeding, and all of them used SurgiGuard® and other products together. Nine patients reported adverse effects, such as abscess, bleeding, or leg swelling, but we found no direct correlation with SurgiGuard®.
Conclusions:
SurgiGuard® exhibited greater effectiveness when used alone. No direct adverse effects associated with SurgiGuard® use were reported, and SurgiGuard® had stable feasibility. Prospective comparative studies are needed in the future.
01 Jan 2023·Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association
Feasibility and safety of inserting transient biodegradable stents in the pylorus during pylorus-preserving gastrectomy for gastric cancer: a preliminary study in a porcine for proof of concept
Article
Author: Yoon, Hyesung ; Choi, Jong-Ho ; Yang, Han-Kwang ; Park, Ji-Hyeon ; Park, Shin-Hoo ; Wang, Chaojie ; Kong, Seong-Ho ; Alzahrani, Fadhel Dhaifallah H ; Kim, Hyun Myong ; Park, Do Joong ; Wang, Sen ; Koo, Eunhee ; Kwak, Yoon Jin ; Lee, Hyuk-Joon ; Yoo, Ja Eun ; Alzahrani, Khalid Mohammed
BACKGROUND:
To evaluate whether insertion of self-biodegradable stent into the pylorus to prevent delayed-gastric emptying after pylorus-preserving gastrectomy is feasible and safe through porcine experiment.
METHODS:
Self-biodegradable dumbbell-shaped pyloric stents were designed from absorbable suture materials: poly(glycolide-co-caprolactone) (PGCL) or poly-p-dioxanone (PPDO). After gastrotomy on ten pigs, each stent was inserted: two shams, four PGCL stents, and four PPDO stents. Body weight (Bwt), body temperature (BT), complete blood cell (CBC) count, and plain X-ray were evaluated. On postoperative day (POD) 13, euthanasia was performed for histologic evaluation.
RESULTS:
Operation was successfully performed in all ten pigs. Without tagging suture, both stents migrated before POD 3. The migration was delayed up to POD 13, when the tagging sutures (-t) were applied between stent and stomach wall. Self-degradation of PGCL started from POD 3, and stents were completely excreted from the abdomen by POD 8. Although PPDO were also weakened as self-degradation progressed, its shape was maintained in gastrointestinal tract for 13 days. Unexpected sudden death occurred in the pig with PPDO-t2 on POD 10, which is more likely due to acute volvulus rather than stent-related complication. There was no significant difference between three groups in terms of Bwt, BT, CBC, and histology (sham vs. PGCL vs. PPDO, all p > 0.05).
CONCLUSION:
The concept of biodegradable stents made of absorbent suture material seems feasible in porcine experiment. Among them, PGCL which has shown rapid absorption, appears to be a more suitable material for transient pyloric absorbable stent when considering safety aspect.
01 Jan 2022·The Korean journal of physiology & pharmacology : official journal of the Korean Physiological Society and the Korean Society of PharmacologyQ4 · MEDICINE
Gallic acid-mitochondria targeting sequence-H3R9 induces mitochondria-targeted cytoprotection
Q4 · MEDICINE
Article
Author: Ko, Kyung Soo ; Kim, Goo-Young ; Han, Jin ; Jessa, Flores ; Bae, Yoonhee
The development of selective targeting of drug molecules towards the mitochondria is an important issue related to therapy efficacy. In this study, we report that gallic acid (GA)-mitochondria targeting sequence (MTS)-H3R9 exhibits a dual role as a mitochondria-targeting vehicle with antioxidant activity for disease therapy. In viability assays, GA-MTS-H3R9 showed a better rescue action compared to that of MTS-H3R9. GA-MTS-H3R9 dramatically exhibited cell penetration and intercellular uptake compared to MTS and fit escape from lysosome release to the cytosol. We demonstrated the useful targeting of GA-MTS-H3R9 towards mitochondria in AC16 cells. Also, we observed that the antioxidant properties of mitochondrial-accrued GA-MTSH3R9 alleviated cell damage by reactive oxygen species production and disrupted mitochondrial membrane potential. GA-MTS-H3R9 showed a very increased cytoprotective effect against anticancer activity compared to that of MTS-H3R9. We showed that GA-MTS-H3R9 can act as a vehicle for mitochondria-targeting and as a reagent for therapeutic applications intended for cardiovascular disease treatment.
7
News (Medical) associated with Samyang Biopharmaceuticals Corp.29 Nov 2022
DUBLIN--(
BUSINESS WIRE
)--The
"Surgical Devices Pipeline Report including Stages of Development, Segments, Region and Countries, Regulatory Path and Key Companies, 2022 Update"
report has been added to
ResearchAndMarkets.com's
offering.
This report provides comprehensive information about the Surgical Devices pipeline products with comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
Report Scope
Extensive coverage of the Surgical Devices under development
The report reviews details of major pipeline products which includes, product description, licensing and collaboration details and other developmental activities
The report reviews the major players involved in the development of Surgical Devices and list all their pipeline projects
The coverage of pipeline products based on various stages of development ranging from Early Development to Approved/Issued stage
The report provides key clinical trial data of ongoing trials specific to pipeline products
Recent developments in the segment/industry
The report enables you to:
Formulate significant competitor information, analysis, and insights to improve R&D strategies
Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage
Identify and understand important and diverse types of Surgical Devices under development
Develop market-entry and market expansion strategies
Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
In-depth analysis of the product's current stage of development, territory and estimated launch date
Key Topics Covered
1 Table of Contents
1.1 List of Tables
1.2 List of Figures
2 Introduction
2.1 Surgical Devices Overview
3 Products under Development
3.1 Surgical Devices - Pipeline Products by Stage of Development
3.2 Surgical Devices - Pipeline Products by Segment
3.3 Surgical Devices - Pipeline Products by Territory
3.4 Surgical Devices - Pipeline Products by Regulatory Path
3.5 Surgical Devices - Pipeline Products by Estimated Approval Date
3.6 Surgical Devices - Ongoing Clinical Trials
4 Surgical Devices - Pipeline Products under Development by Companies
4.1 Surgical Devices Companies - Pipeline Products by Stage of Development
4.2 Surgical Devices - Pipeline Products by Stage of Development
5 Surgical Devices Companies and Product Overview
6 Surgical Devices - Recent Developments
7 Appendix
7.1 Methodology
List of Tables
Surgical Devices - Pipeline Products by Stage of Development
Surgical Devices - Pipeline Products by Segment
Surgical Devices - Pipeline Products by Territory
Surgical Devices - Pipeline Products by Regulatory Path
Surgical Devices - Pipeline Products by Estimated Approval Date
Surgical Devices - Ongoing Clinical Trials
Surgical Devices Companies - Pipeline Products by Stage of Development
Surgical Devices - Pipeline Products by Stage of Development
Glossary
List of Figures
Surgical Devices - Pipeline Products by Stage of Development
Surgical Devices - Pipeline Products by Segment
Surgical Devices - Pipeline Products by Territory
Surgical Devices - Pipeline Products by Regulatory Path
Surgical Devices - Pipeline Products by Estimated Approval Date
Surgical Devices - Ongoing Clinical Trials
Companies Mentioned
6S Medical LLC
Abbott Operations Uruguay Srl
Acoustic MedSystems Inc
Aesculap Inc
Anastom Surgical
Anchora Medical Ltd
Apercure Surgical Ltd
Apollo Endosurgery Inc
Applied Medical Resources Corporation
Apyx Medical Corp
Archon Medical Technologies LLC
Augusta University
Baxter International Inc
BioTex Inc
BriteSeed
BTG International Inc
Carnegie Mellon University
Centre Hospitalier Universitaire de Nice
Charite University Hospital of Berlin
Chengdu Yurui Innovation Technology Co Ltd
Colospan Ltd
Control Medical Technology LLC
Core Essence Orthopaedics, LLC
Corporis Medical
Covidien Ltd
CR Bard Inc
Creative Balloons GmbH
Cryofocus Medtech Shanghai Co Ltd
CT Resources Inc
Cutera Inc
Cypris Medical, Inc.
Dartmouth College
Deep Blue Medical Advances Inc
Drexel University
Emblation Ltd
Embricon Ltd (Inactive)
Endo Tools Therapeutics SA
EndoEvolution, LLC
Ethicon Endo-Surgery Inc
ExpandoHeat LLC
Fixit Medical Ltd
Fogless International AB
G.I. Windows Inc
Galil Medical Ltd
Garantis
Genicon Inc
GI Windows Medical Corp
Grand Valley State University
H. Lee Moffitt Cancer Center & Research Institute Inc
Hangzhou Kangsheng Medical Equipment Co Ltd
Hannover Medical School
Harvard University
Hospital for Special Surgery
IceCure Medical Ltd
Imperial College London
Indian Institute of Technology Delhi
Innovex Medical Co Ltd
Integra LifeSciences Holdings Corp
Intuitive Surgical Inc
IonMed Ltd.
Jinan Micro Intelligent Technology Co Ltd
Johns Hopkins University
Keren Medical Ltd.
LeMaitre Vascular Inc
Livsmed Inc
LuSeed Vascular
Lymphatica Medtech SA
MagniFeel Surgical Instruments
Mayo Clinic
Mecmaan Healthcare Ltd
Medical University of South Carolina
MediShield B.V.
Medtronic Plc
Mel Frontier Co Ltd
Meta Biomed Co Ltd
Michigan State University
Naval Medical Research Center
Next Science Ltd
NorthShore University Health System
NOvate Medical Technologies, LLC
NoviRad Inc
NovoGI LTD
Novolock Ltd
Novuson Surgical, Inc.
Ohio State University
Oklahoma State University
Olympus America Inc
OV World Co Ltd
Pacira BioSciences Inc
Parker Hannifin Corp
Pavmed Inc
PetVivo Holdings Inc
Physcient, Inc.
Polytechnic University of Catalonia
Pro-Dex Inc
Queen Mary University of London
Ra Medical Systems Inc
Ramona Optics Inc
Ratner BioMedical Inc
Resultados y Calidad del Sistema Sanitario Publico de Andalucia
Rice University
Saint Louis University
Samyang Biopharmaceuticals Corp
Sanovas Inc
Shanghai MicroPort Medical Group Co Ltd
Shanghai Shengzhe Medical Technology Co Ltd
Shanghai Yichao Medical Equipment Co Ltd
Silmag SA
simedeq
Simon Fraser University
StatLink Surgical
STRATA Skin Sciences Inc
Stryker Corp
SurgyAid, LLC
Sutura Inc
Teleflex Inc
Tepha Inc
The Feinstein Institute for Medical Research
TransMed7 LLC
Trinity College Dublin
University of Auckland
University of California
University of California Davis
University of California Los Angeles
University of California San Diego
University of Central Florida
University of Chicago
University of Maryland
University of Maryland Baltimore
University of Minnesota
University of Missouri
University of North Carolina
University of South Florida
University of Strasbourg
University of Texas at Austin
University of Texas Medical Branch at Galveston
University of Toledo
University of Utah
University of Virginia
US Medical Innovations LLC
USGI Medical Inc
Vanderbilt University
Vascular Insights LLC
VB Devices
Vessi Medical Ltd
Viveve Medical Inc
Viveve, Inc.
Wake Forest Baptist Medical Center
Weill Cornell Medical College
West Virginia University
X-Biomedical Inc
For more information about this report visit
https://www.researchandmarkets.com/r/ekyrrp
Source: GlobalData
16 Nov 2022
Participated in 'IMCAS Academy' webinar hosted by the IMCAS for 3 consecutive years
Brazilian dermatologists announced the superiority of Croquis, actively targeting the Latin American market
Pre-registered and recorded the most significant number of simultaneous viewers with a single topic for the lifting thread
SEOUL, South Korea, Nov. 16, 2022 /PRNewswire/ -- Samyang Holdings Biopharmaceuticals Division's lifting thread brand 'Croquis,' is actively targeting the Latin American market following the EU and expanding its market base.
Samyang Holdings Biopharmaceuticals Division (CEO: Lee Young-Joon) announced on November 2nd that it held a webinar at the 'IMCAS Academy' to inform the safety and efficacy of Croquis and introduced the procedure. International Master Course on Aging Science (IMCAS) is one of the world's three major medical aesthetics conferences, where the experts share the latest trends and medical technology. The IMCAS Academy is an online lecture platform.
Samyang Holdings presented the lifting procedure with medical experts under the 'Bio Stimulation & Lifting Effect with PDO* THREADS' at IMCAS. It received positive responses from the audience when announcing side effects, causes, preventions, and treatment methods.
Samyang Holdings presented three Brazilian dermatologists 'Dr. Clara Santos,' 'Dr. Patrica Omiga,' and 'Dr. Fabiano Leal' as speakers to focus on the Latin American market. They are regarded and respected as influential specialists in the Brazilian medical aesthetics market based on their various clinical experiences. According to the Korea Trade-Investment Promotion Agency (KOTRA), Latin America is an emerging market that shows a significant growth rate every year.
About 1,360 people pre-registered for the Croquis' webinar, and with 435 people simultaneously watching in real-time. This was the most significant number of people attending IMCAS Academy webinars specifically on the lifting thread, which confirmed the high interest for Croquis.
Samyang Holdings announced global case study results for the lifting thread brand 'Croquis', proving its efficacy and safety with data. It signed sales contracts in 18 countries worldwide, including the EU, Japan, and Latin America. It is also in the process of obtaining regulatory marketing approval in the US.
Croquis is gaining the trust of the global medical aesthetics industry by using data-oriented marketing. It is securing various data through clinical trials to prove the correlation between the effect of their treatments and the physical properties of the lifting thread for evidence-based marketing.
SOURCE Samyang Holdings Corp.
Clinical Result
04 Jul 2022
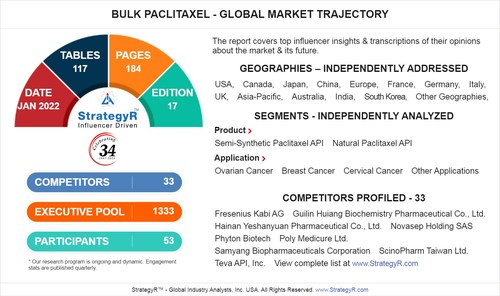
SAN FRANCISCO, July 4, 2022 /PRNewswire/ -- A new market study published by Global Industry Analysts Inc., (GIA) the premier market research company, today released its report titled "Bulk Paclitaxel - Global Market Trajectory & Analytics". The report presents fresh perspectives on opportunities and challenges in a significantly transformed post COVID-19 marketplace.
FACTS AT A GLANCE
What's New for 2022?
With Market Size Valued at $185.4 Million by 2026, it`s a Healthy Outlook for the Global Bulk Paclitaxel Market
Global competitiveness and key competitor percentage market shares
Market presence across multiple geographies - Strong/Active/Niche/Trivial
Online interactive peer-to-peer collaborative bespoke updates
Access to our digital archives and MarketGlass Research Platform
Complimentary updates for one year
Edition: 17;
Released: January 2022
Executive Pool: 1333
Companies: 33 - Players covered include Fresenius Kabi AG; Guilin Huiang Biochemistry Pharmaceutical Co., Ltd.; Hainan Yeshanyuan Pharmaceutical Co., Ltd.; Novasep Holding SAS; Phyton Biotech; Poly Medicure Ltd.; Samyang Biopharmaceuticals Corporation; ScinoPharm Taiwan Ltd.; Teva API, Inc.; Yunnan Hande Bio-tech Co., Ltd./Hande Bio-Source, Inc. (HBS) and Others.
Coverage: All major geographies and key segments
Segments: Product (Semi-Synthetic Paclitaxel API, Natural Paclitaxel API); Application (Ovarian Cancer, Breast Cancer, Cervical Cancer, Other Applications)
Geographies: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Complimentary Project Preview - This is an ongoing global program. Preview our research program before you make a purchase decision. We are offering a complimentary access to qualified executives driving strategy, business development, sales & marketing, and product management roles at featured companies. Previews provide deep insider access to business trends; competitive brands; domain expert profiles; and market data templates and much more. You may also build your own bespoke report using our MarketGlass™ Platform which offers thousands of data bytes without an obligation to purchase our report.
Preview Registry
ABSTRACT-
Global Bulk Paclitaxel Market to Reach $185.4 Million by 2026
Amid the COVID-19 crisis, the global market for Bulk Paclitaxel estimated at US$98.2 Million, is projected to reach a revised size of US$185.4 Million by 2026, growing at a CAGR of 10.9% over the analysis period. Natural Paclitaxel API, one of the segments analyzed in the report, is projected to record a 10.3% CAGR and reach US$68.8 Million by the end of the analysis period. After a thorough analysis of the business implications of the pandemic and its induced economic crisis, growth in the Semi-Synthetic Paclitaxel API segment is readjusted to a revised 11.2% CAGR for the next 7-year period.
The U.S. Market is Estimated at $29.6 Million in 2021, While China is Forecast to Reach $38.3 Million by 2026
The Bulk Paclitaxel market in the U.S. is estimated at US$29.6 Million in the year 2021. China, the world`s second largest economy, is forecast to reach a projected market size of US$38.3 Million by the year 2026 trailing a CAGR of 14.3% over the analysis period. Among the other noteworthy geographic markets are Japan and Canada, each forecast to grow at 7.6% and 9.5% respectively over the analysis period. Within Europe, Germany is forecast to grow at approximately 8.6% CAGR.
More
MarketGlass™ Platform
Our MarketGlass™ Platform is a free full-stack knowledge center that is custom configurable to today`s busy business executive`s intelligence needs! This influencer driven interactive research platform is at the core of our primary research engagements and draws from unique perspectives of participating executives worldwide. Features include - enterprise-wide peer-to-peer collaborations; research program previews relevant to your company; 3.4 million domain expert profiles; competitive company profiles; interactive research modules; bespoke report generation; monitor market trends; competitive brands; create & publish blogs & podcasts using our primary and secondary content; track domain events worldwide; and much more. Client companies will have complete insider access to the project data stacks. Currently in use by 67,000+ domain experts worldwide.
Our platform is free for qualified executives and is accessible from our website or via our just released mobile application on iOS or Android
About Global Industry Analysts, Inc. & StrategyR™
Global Industry Analysts, Inc., () is a renowned market research publisher the world`s only influencer driven market research company. Proudly serving more than 42,000 clients from 36 countries, GIA is recognized for accurate forecasting of markets and industries for over 33 years.
CONTACTS:
Zak Ali
Director, Corporate Communications
Global Industry Analysts, Inc.
Phone: 1-408-528-9966
Email: [email protected]
LINKS
Join Our Expert Panel
Connect With Us on LinkedIn
Follow Us on Twitter
Journalists & Media
[email protected]
SOURCE Global Industry Analysts, Inc.
Collaborate
100 Deals associated with Samyang Biopharmaceuticals Corp.
Login to view more data
100 Translational Medicine associated with Samyang Biopharmaceuticals Corp.
Login to view more data
Corporation Tree
Boost your research with our corporation tree data.
login
or

Pipeline
Pipeline Snapshot as of 23 Feb 2026
The statistics for drugs in the Pipeline is the current organization and its subsidiaries are counted as organizations,Early Phase 1 is incorporated into Phase 1, Phase 1/2 is incorporated into phase 2, and phase 2/3 is incorporated into phase 3
Other
23
Login to view more data
Current Projects
| Drug(Targets) | Indications | Global Highest Phase |
|---|---|---|
Docetaxel polymeric micelle ( Tubulin ) | Head and Neck Neoplasms More | Withdrawn |
B-5626 | Lung Cancer More | Withdrawn |
Capsaicin ( PRDX2 x TRPV1 ) | Peripheral Nerve Injuries More | Discontinued |
SYO-1644 | Neoplasms More | Discontinued |
SYP-1261 | Kidney Neoplasms More | Discontinued |
Login to view more data
Deal
Boost your decision using our deal data.
login
or

Translational Medicine
Boost your research with our translational medicine data.
login
or

Profit
Explore the financial positions of over 360K organizations with Synapse.
login
or

Grant & Funding(NIH)
Access more than 2 million grant and funding information to elevate your research journey.
login
or

Investment
Gain insights on the latest company investments from start-ups to established corporations.
login
or

Financing
Unearth financing trends to validate and advance investment opportunities.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free